Trichoderma biocontrol recombinant engineering bacteria for efficiently expressing chitinase coding gene and Beta-1,3-glucanase coding gene as well as application thereof
A technology of recombinant engineering bacteria and chitinase, applied in genetic engineering, application, plant genetic improvement and other directions, can solve the problems of poor biocontrol effect, undiscovered, unstable functional gene content, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
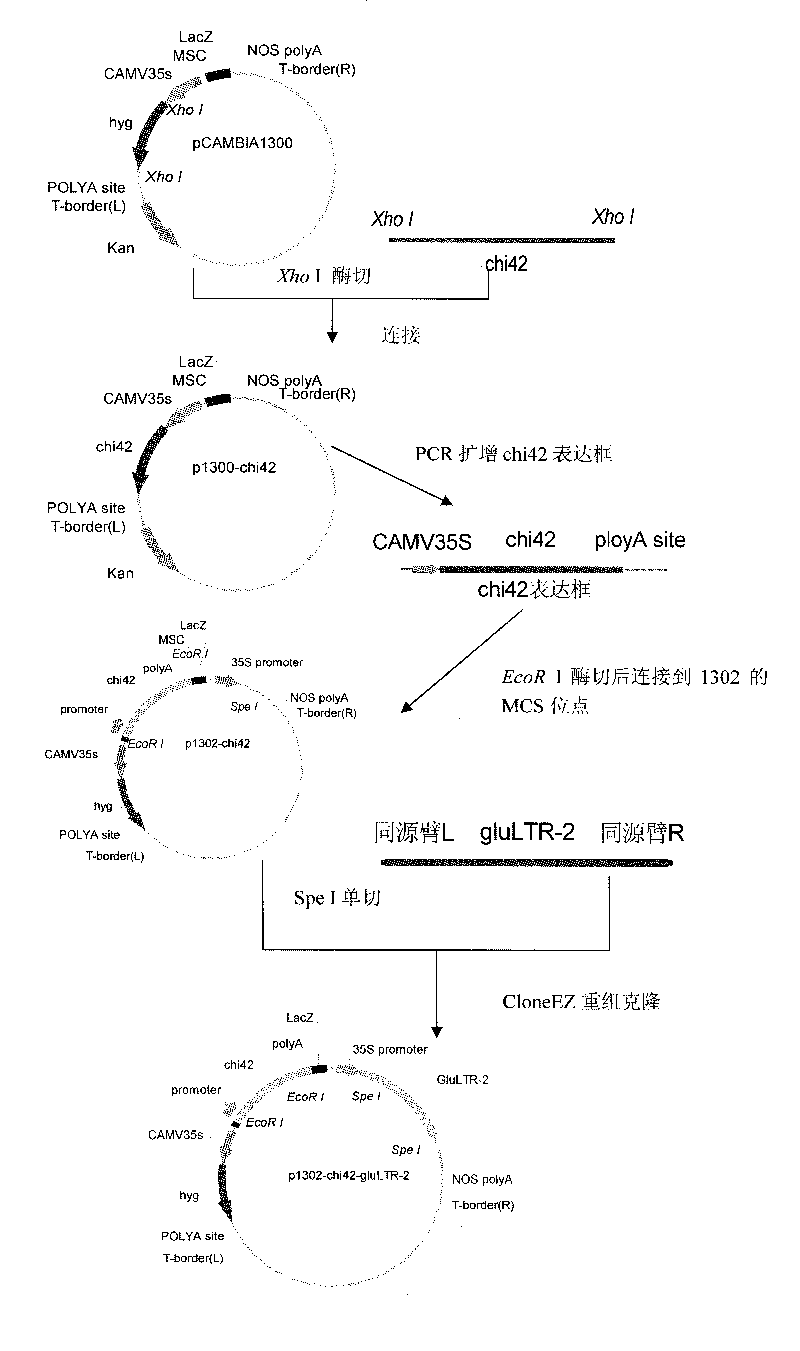
[0037] Example 1: Cloning of Trichoderma chitinase Chi42 and β-1,3-glucanase encoding genes glultr-2
[0038] Inoculate Trichoderma viride LTR-2 conidia in PDB medium (the formula is: potato 200g; glucose 15g; deionized water to make the volume to 1000mL, peel the potato, cut into small pieces, add 1000mL water to the pot Boil for half an hour, filter with double gauze, take the filtrate, add sugar, and add water to make up to 1000mL), culture for 48-72h, extract genomic DNA, and check DNA purity and quantification by electrophoresis. Using genomic DNA as template, using the upstream primer Chi42XhoI-F: 5’GC CTCGAG ATGTTGGGCTTCCTCGG 3'(the underlined part is the XhoI restriction site) downstream primer Chi42XhoI-R: 5'GC CTCGAG CTTGTCGAACAAGCTTCTAGTTGAGACC 3'(the underlined part is the XhoI restriction site) for PCR amplification. Add 5μL of 10×Taq DNA polymerase buffer to the 50μL reaction system, the upstream and downstream primers to 20μM, dNTP 100μM, MgCl2 1mM, template DNA ...
Embodiment 2
[0040] Example 2: Construction of p1302-chi42
[0041] The CaMV35S promoter and terminator of the hygromycin gene hph of pCAMBIA1300 were used as the 42kDa chitinase gene promoter and terminator, and then inserted into the expression vector pCAMBIA1302. Constructed to obtain p1302-chi42. The specific method is as follows:
[0042] The first is the connection between the plasmid pCAMBIA1300 and the chitinase gene. The PCR amplification product of the 42kDa chitinase gene contains XhoI restriction sites at both ends. The PCR amplification product is directly digested with XhoI and then digested with XhoI. The dephosphorylated pCAMBIA1300 vector was ligated with T4 ligase and transformed into E. coli. Due to the problem of forward and reverse for single enzyme digestion, we use PCR and SacI digestion methods. Forward connection, the sequence between the two restriction sites is about 2.4kb, and the reverse connection is about 1.1kb. The positive clones screened by SacI digestion PC...
Embodiment 3
[0043] Example 3: Construction of p1302-chi42-glultr-2
[0044] The recombinant plasmid pMD18-T-glultr-2 was used as a template for PCR amplification. The 50μL PCR reaction system is: 10×pyrobestbufferII 5μL, upstream and downstream primers 20μM, dNTP 100μM, MgCl2 1mM, template 1μL, pyrobest DNA Polymerase 0.5μL, ddH 2 Make up to 50 μL of O. The primer sequence is as follows
[0045] gluEZF5′ CTCTTGACCATGGTAGATCTG ACTAGTATGTTGAAGCTCACGGCGCTCG
[0046] (The underlined part is the same nucleic acid sequence as one end of the pCAMBIA1302 vector linearized by SpeI)
[0047] gluEZR5′ GTGAAAAGTTCTTCTCCTTT ACTAGTAGTAGTATAACGGGCAACGTCACCACCTCC (the underlined part is the same nucleic acid sequence as the other end of the pCAMBIA1302 vector linearized by SpeI). The reaction conditions are: 94°C pre-denaturation for 5 minutes; 94°C denaturation for 1 minute, 58°C annealing for 1.5 minutes, 72°C extension for 2 minutes, 30 cycles; Extension at 72°C for 10 min. Recover PCR products and use ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com