Cyanine dye having azulene structure and preparation method thereof
A technology of cyanine dyes and alkyl groups, applied in the field of azulene-containing structural cyanine dyes and their preparation, can solve the problems of low conversion rate, poor reaction selectivity, difficult purification and the like, and achieves high conversion rate, good selectivity and easy purification. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
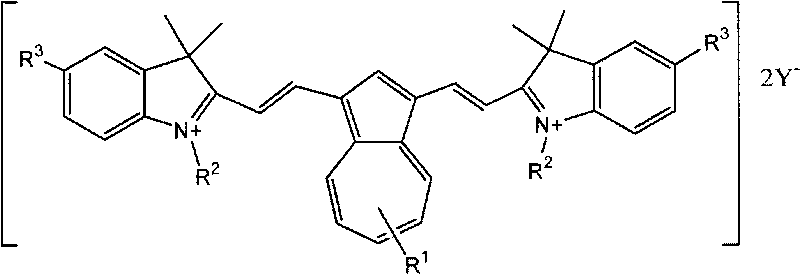
[0043] The structural formula of the synthetic product:
[0044]
[0045] In a 250mL three-necked reaction flask with a mechanical stirrer and a condenser, add 1,3-diformyl azulene (18.4 g, 0.1 mol), 1-ethyl-2,3,3-trimethylindole iodide Salt (81.9 g, 0.26 mol) and acetic anhydride (100 mL), heated to reflux for 5 hours, cooled to room temperature, added water (200 mL) and stirred for 2 hours, and filtered the precipitated crystals.
[0046] The obtained crude product was dried, recrystallized with methanol, and filtered to obtain 57.6 g of green crystals with a yield of 74%.
[0047] The structure of the product was confirmed by NMR absorption:
[0048] 1H NMR (400MHz, DMSO-d 6 ): 1.42(t, 6H), 2.03(s, 12H), 4.26(m, 4H), 7.02(d, 2H), 7.11-7.20(m, 6H), 7.31-7.35(m, 2H), 7.54- 7.56 (m, 1H), 7.60 (d, 2H), 8.13 (s, 1H), 8.16 (d, 2H), 8.47 (d, 2H).
Embodiment 2
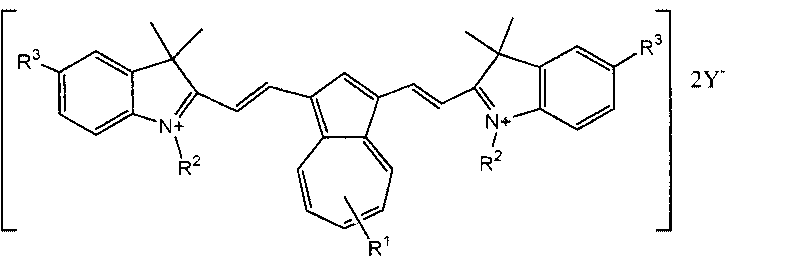
[0050] The structural formula of the synthetic product:
[0051]
[0052]In a 250mL three-necked reaction flask with a mechanical stirrer and a condenser, add 1,3-diformyl azulene (18.4 grams, 0.1 mol), 1-butyl-2,3,3-trimethylindole bromide Substitute salt (88.8 g, 0.3 mol) and acetic anhydride (150 mL), heated to reflux for 8 hours, cooled to room temperature, stirred, added water (250 mL), and filtered the precipitated crystals.
[0053] The obtained crude product was dried, recrystallized with ethanol, potassium fluoroborate (37.8 g) was added at the same time, heated to reflux for 2 hours, hot filtered, the filtrate was cooled to room temperature, and the precipitated crystals were filtered to obtain 50.0 g of green crystals, yield 63%.
[0054] The structure of the product was confirmed by NMR absorption:
[0055] 1H NMR (400MHz, DMSO-d 6 ): 0.94(t, 6H), 1.45(m, 4H), 1.76(m, 4H), 1.98(s, 12H), 4.22(t, 4H), 7.08(d, 2H), 7.18-7.29(m, 6H), 7.35-7.39 (m, 2H), 7.50-7.57...
Embodiment 3
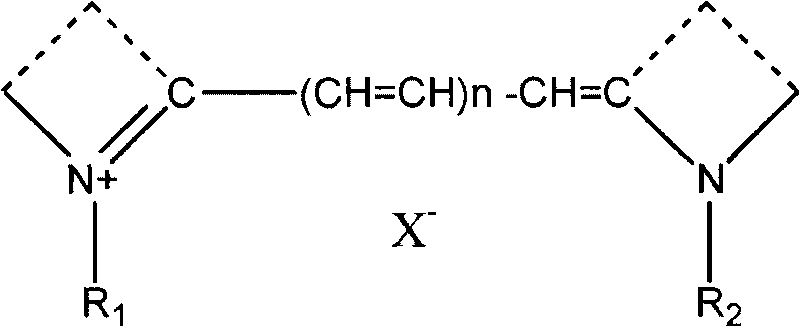
[0057] The structural formula of the synthetic product:
[0058]
[0059] In a 250mL three-necked reaction flask with a mechanical stirrer and a condenser, add 1,3-diformyl-2,4,6-trimethylazulene (22.6 grams, 0.1 mol), 1-ethyl-2, 3,3-Trimethylindole iodide (113.4 g, 0.36 mol), pyridine (1 g), tert-butanol (100 mL) and benzene (50 mL), stirred, heated to reflux for 9 hours, cooled to room temperature, filtered to precipitate crystallization.
[0060] The obtained crude product was dried, recrystallized with ethanol, and potassium hexafluorophosphate (66 g) was added at the same time, heated to reflux for 1 hour, cooled to room temperature, and the precipitated crystals were filtered to obtain 58.2 g of green crystals with a yield of 68%.
[0061] The structure of the product was confirmed by NMR absorption:
[0062] 1H NMR (400MHz, DMSO-d 6 ): 1.50(t, 6H), 2.13(s, 12H), 2.67(s, 3H), 3.05(s, 6H), 4.28(m, 4H), 7.08(d, 2H), 7.14-7.26(m, 6H), 7.52 (s, 1H), 7.54 (d, 2H), 8.15...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com