Synthetic method of (S)-1,2,3,4-tetrahydroisoquinoline tert-butyrylamide with high optical purity
A technology of tetrahydroisoquinoline tert-butyramide and optical purity, applied in the direction of organic chemistry and the like, can solve the problems of low total yield, long reaction steps and high production cost, and achieve the effects of low raw material cost and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
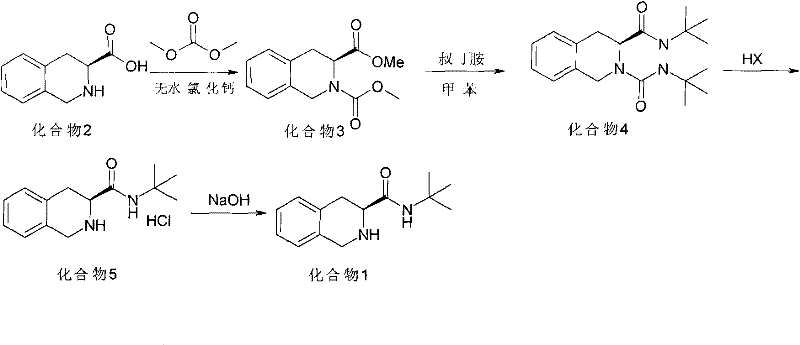
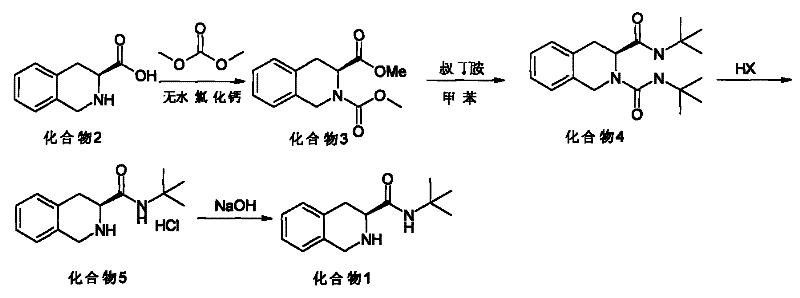
[0021] Such as figure 1 Shown, the synthetic method of high optical purity (S)-1,2,3,4-tetrahydroisoquinoline tert-butyramide, with (S)-1,2,3,4-tetrahydroisoquinoline carboxylic acid As a raw material, dimethyl carbonate is used to synthesize (S)-N-methyl formate-1,2,3,4-tetrahydroisoquinoline carboxylate methyl ester, and to obtain ureide by aminolysis, removal of ureido group, alkalization, Obtain (S)--1,2,3,4-tetrahydroisoquinoline tert-butyramide, specifically comprising the following steps:
[0022] ①Quinoline carboxylic acid compound (2) is reacted with dimethyl carbonate to prepare compound (3) of N-methyl formate; the catalyst is a halogenated salt, the solvent is dimethyl carbonate, and the reaction temperature is 0°C to 95°C;
[0023] ② Compound (3) is subjected to aminolysis with tert-butylamine to prepare compound (4); the solvent is an inert solvent of aromatic hydrocarbon or halogenated alkanes, and the reaction temperature is 20°C to 160°C;
[0024] ③The compo...
Embodiment 1
[0030] Step 1 Put 38.4 g of S-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (compound 2), 200 ml of dimethyl carbonate, and 23 g of anhydrous CaCl into a reaction flask. The reaction was stirred at room temperature for 30 minutes, and the temperature was raised to reflux for 2 hours. After the reaction was completed, dimethyl carbonate was concentrated under reduced pressure, and about 50ml remained, and 50ml of water was slowly added dropwise, cooled to 0°C, stirred for 30 minutes, filtered with suction to obtain a light yellow solid, and dried to obtain about S-N-methyl formate-1,2, 45 g of methyl 3,4-tetrahydroisoquinoline-3-carboxylate (compound 3). HPLC purity: 99.0%, yield: 85%.
[0031] Step 2 Add 26.4g of S-N-methyl formate-1,2,3,4-tetrahydroisoquinoline-3-carboxylate (compound 3), 300ml of toluene, and 20g of tert-butylamine into a 1000ml autoclave, and replace with nitrogen Air in the kettle. Stir and heat up to 100°C, and react for 18 hours. Cool to room temp...
Embodiment 2
[0035] Step 1 Put 38.4 g of S-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (compound 2), 200 ml of dimethyl carbonate, and 3.2 g of MgCl2 into a reaction flask. The reaction was stirred at room temperature for 30 minutes, and the temperature was raised to reflux for 10 hours. After the reaction was completed, dimethyl carbonate was concentrated under reduced pressure, and about 50ml remained, and 50ml of water was slowly added dropwise, cooled to 0°C, stirred for 30 minutes, filtered with suction to obtain a light yellow solid, and dried to obtain about S-N-methyl formate-1,2, 48 g of methyl 3,4-tetrahydroisoquinoline-3-carboxylate (compound 3). HPLC purity: 98.7%, yield: 90%.
[0036] Step 2 Add 26.4g of S-N-methyl formate-1,2,3,4-tetrahydroisoquinoline-3-carboxylate (compound 3), 200ml of dichloroethane, and 20g of tert-butylamine into a 1000ml autoclave , Nitrogen replaced the air in the kettle. Stir and heat up to 80°C, and react for 10 hours. Cool to room temperat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com