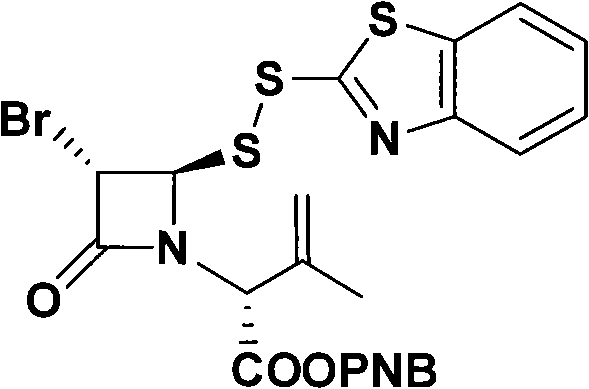
Synthesis method of single-ring imipenem p-nitro benzyl ester
A technology of p-nitrobenzyl ester and synthesis method, which is applied in the field of synthesis of monocyclopenem p-nitrobenzyl ester, and can solve problems such as poor product quality, complicated operation, and serious pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
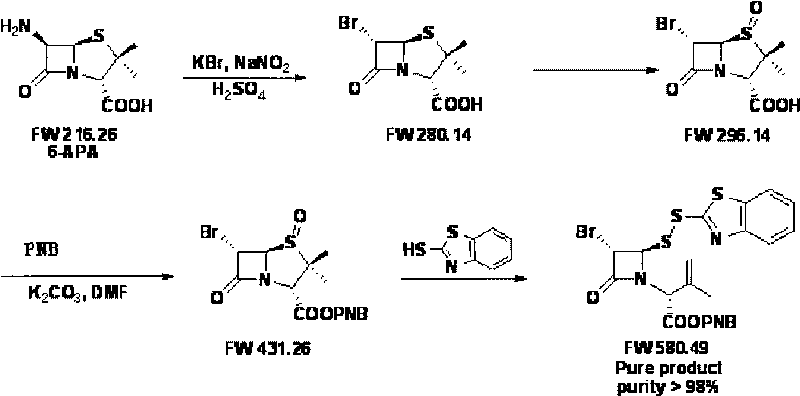
[0038] (1) Synthesis of 6α-bromo-penicillanic acid (bromo)
[0039] In a 1000-liter reactor, add 470 kg of water, cool down to 0-5°C, and add 35 kg of 98% sulfuric acid. After the addition, 120.2 kg of potassium bromide, 43.2 kg of 6-APA and 120 kg of toluene were added successively when the temperature dropped below -10°C. After the addition, wait until the reaction temperature drops below -10°C, start adding 100 kg of 20% sodium nitrite aqueous solution dropwise, and control the reaction temperature at -15 to -10°C for 1 hour. After the dropwise addition, the reaction was carried out under temperature control and stirring for 4 hours, and the liquid phase detection of 6-APA was less than 0.5%. Add 150 kg of toluene, stir and react at below 10°C for 30 minutes, filter, separate layers, extract the aqueous phase with 50 kg of toluene, combine the organic phases and wash with 50 kg of saturated saline, and extract the aqueous phase with 20 kg of toluene after the organic phase...
Embodiment 2
[0047] (1) Synthesis of 6α-bromo-penicillanic acid (bromo)
[0048] In a 1000-liter reactor, add 400 kg of water, cool down to 0-5°C, and add 35 kg of 98% sulfuric acid. After the addition, 120.2 kg of potassium bromide, 43.2 kg of 6-APA and 120 kg of toluene were added successively when the temperature dropped below -10°C. After the addition, wait for the reaction temperature to drop below -10°C, start to add 21.2kg of sodium nitrite in 100kg of aqueous solution dropwise, and control the reaction temperature to -13~-15°C for 1.5h. After the dropwise addition, the reaction was carried out under temperature control and stirring for 2 hours, and the liquid phase detection of 6-APA was less than 0.5%. Add 150 kg of toluene, stir for 30 min below 10°C, filter, separate layers, extract the aqueous phase with 30 kg of toluene, combine the organic phases and wash with 50 mg of saturated brine, and extract the aqueous phase with 20 kg of toluene after the organic phases are separated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com