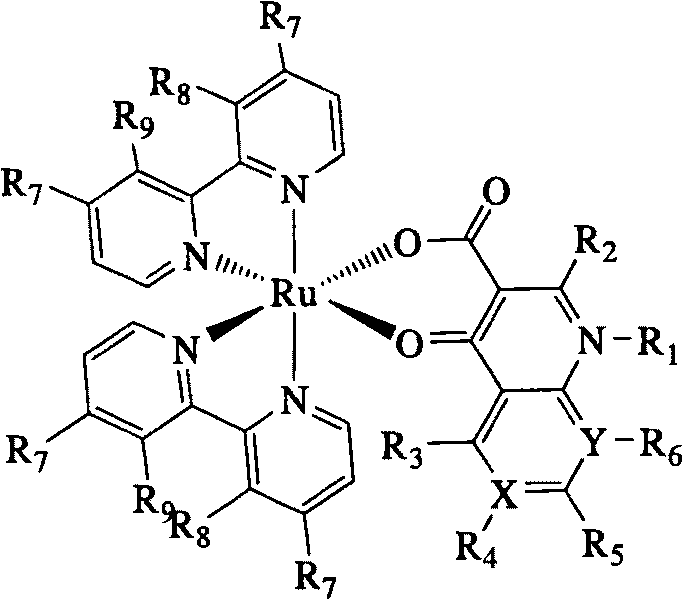
Ruthenium polypyridyl complex using quinolones compound as ligand, preparation method and application thereof
A technology of ruthenium polypyridine and quinolones, which is applied in the field of metal complexes of quinolones, can solve the problems of prolonging the survival time of tumor patients, achieve good inhibition effect, improve lipid-water partition coefficient, and enhance absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 [Ru(bpy) with ofloxacin (Ofloxacin) as a ligand 2 Preparation of OFLX]Cl
[0030]
[0031] 1.1 cis-[Ru(bpy) 2 Cl 2 ]·2H 2 Synthesis of O
[0032] Ruthenium trichloride (RuCl 3 ·nH 2 O) (1.56g, 6mmol), bipyridine (1.87g, 12mmol) and lithium chloride (2.42g, 57.6mmol), were dissolved in 15ml of DMF, and heated to reflux for 8 hours under the protection of argon. Cool to room temperature, add 50ml of acetone, shake well, and freeze overnight in the refrigerator. After filtration, purple-black microcrystals were obtained, which were washed with ice water until nearly colorless, and dried, with a yield of 71.0% (calculated as bipyridine).
[0033] 1.2 [Ru(bpy) 2 Preparation of OFLX]Cl
[0034] Add cis-[Ru(bpy) to the 100ml three-necked bottle 2 ] Cl 2 2H 2 O (104mg, 0.2mmol), ofloxacin (100mg 0.3mmol), sodium ethoxide (34mg, 0.5mmol), 50ml of absolute ethanol were stirred and dissolved, refluxed at 100°C for 3h under the protection of argon, and an e...
Embodiment 2
[0036] Example 2 [Ru(phen) with ofloxacin (Ofloxacin) as a ligand 2 Preparation of OFLX]Cl
[0037]
[0038] 2.1 cis-Ru(phen) 2 Cl 2 2H 2 Synthesis of O
[0039] RuCl 3 ·nH 2 O, phenanthroline (2.16g, 12mmol), LiCl (1.68g, 28mmol), DMF (10ml) was added, and refluxed under the protection of argon for 8 hours. After the reactant was cooled to room temperature, acetone (50 ml) was added to the reactant and frozen overnight to obtain purple crystals. Washed with cold water and acetone, and dried in vacuum, the yield was 72%.
[0040] 2.2 [Ru(phen) 2 Preparation of OFLX]Cl
[0041] Add cis-[Ru(phen) 2 ] Cl 2 2H 2O (113mg, 0.2mmol), ofloxacin (100mg, 0.3mmol), sodium ethoxide (34mg, 0.5mmol), and 50ml of absolute ethanol were stirred and dissolved, and returned to 100°C for 3h under the protection of argon. After the reaction was completed, an equivalent amount of Remove excess sodium ethoxide with hydrochloric acid, spin dry to recover the solvent, dissolve the obta...
Embodiment 3
[0043] Example 3 [Ru(dmbpy) with ofloxacin (Ofloxacin) as a ligand 2 Preparation of OFLX]Cl
[0044]
[0045] 3.1 cis-[Ru(dmbpy) 2 Cl 2 ]·2H 2 Synthesis of O
[0046] Ruthenium trichloride (RuCl 3 ·nH 2 O) (1.56g, 6mmol), 4,4'-dimethyl-2,2'-bipyridine (2.21g, 12mmol) and LiCl (2.42g, 57.6mmol) in 15ml DMF, argon Heating to reflux under air protection for 8 hours. Cool to room temperature, add 50ml of acetone, shake well, and freeze overnight in the refrigerator. Filtrate to obtain purple-black microcrystals, wash with ice water until nearly colorless, and dry, with a yield of 70.0% (calculated as bipyridine).
[0047] 3.2 [Ru(dmbpy) 2 Preparation of OFLX]Cl
[0048] Add cis-[Ru(dmbpy) to the 100ml three-necked bottle 2 ] Cl 2 2H 2 O (115mg, 0.2mmol), ofloxacin (100mg, 0.3mmol), sodium ethoxide (34mg, 0.5mmol), and 50ml of absolute ethanol were stirred and dissolved, and refluxed at 100°C for 3h under the protection of argon. After the reaction was completed, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com