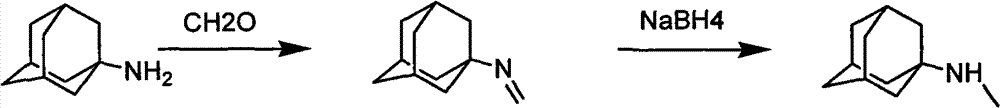
Synthetic method of N-rimantadine
A technique for the synthesis of methylamantadine, which is applied to the preparation of amino compounds, chemical instruments and methods, purification/separation of amino compounds, etc., and can solve problems such as unstable imines and difficulty in separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Put 2g of amantadine hydrochloride raw material into a 50ml round-bottomed flask, add 20mL of ethanol, add 5mL of 37% formaldehyde aqueous solution dropwise for about 5 minutes, and stir magnetically at room temperature. After the addition of formaldehyde, 6M NaOH solution was added dropwise to adjust the pH=10. After 40 minutes, a sample was taken from the reaction system and extracted with water and ethyl acetate. The reaction yield was 85% according to GC analysis. The methyleneation reaction is substantially complete. The yield of the continued reaction does not change much, and the system has reached a dynamic equilibrium.
[0025] Pour the reaction solution into 30mL of ice water, separate it with a separatory funnel, wash the imine with a 33% ethanol aqueous solution at 0-5°C, after washing, add 10mL of ethanol to dissolve the imine, weigh 0.5g of sodium borohydride and add 5mL of water was dissolved, and the reduction reaction was carried out in an ice-water ba...
Embodiment 2
[0028] Add 2g of amantadine hydrochloride raw material into a 50mL round bottom flask, add 20mL of ethanol, add dropwise 7.5mL of 37% formaldehyde aqueous solution in about 5 minutes, and stir magnetically at room temperature. 40 minutes after the addition of formaldehyde, the reaction system was sampled, alkali was added to adjust the pH, the sample was extracted with ethyl acetate and analyzed by GC, and the reaction yield was 55%. The possible reason is that amantadine forms a salt, which reduces the reactivity of nucleophilic addition.
[0029] The reaction product was separated with a separatory funnel, and the obtained imine was washed with 33% ethanol solution at 0-5° C. after washing, the imine was dissolved in 10 mL of ethanol, and 0.5 g of sodium borohydride was weighed and dissolved in 5 mL of water. Under the reduction reaction. After 30 minutes of reaction, a sample was extracted with ethyl acetate and injected into GC for analysis. The content of the by-product ...
Embodiment 3
[0031] Put 2g of newly prepared amantadine raw material into a 50mL round bottom flask, add 20mL of ethanol, add dropwise 7.5mL of 37% formaldehyde aqueous solution in about 5 minutes, and stir magnetically at room temperature. 40 minutes after the addition of formaldehyde was completed, the reaction system was sampled, alkali was added to adjust the pH, the sample was extracted with ethyl acetate and analyzed by GC, and the reaction yield was 85%. The possible reason is the high reactivity of free amantadine. However, amantadine is easy to form carbonate in the air, which affects the normal progress of the reaction.
[0032] The reaction product was separated with a separatory funnel, and the obtained imine was washed with 33% ethanol solution at 0-5° C. after washing, the imine was dissolved in 10 mL of ethanol, and 0.5 g of sodium borohydride was weighed and dissolved in 5 mL of water. Under the reduction reaction. After 30 minutes of reaction, a sample was extracted with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com