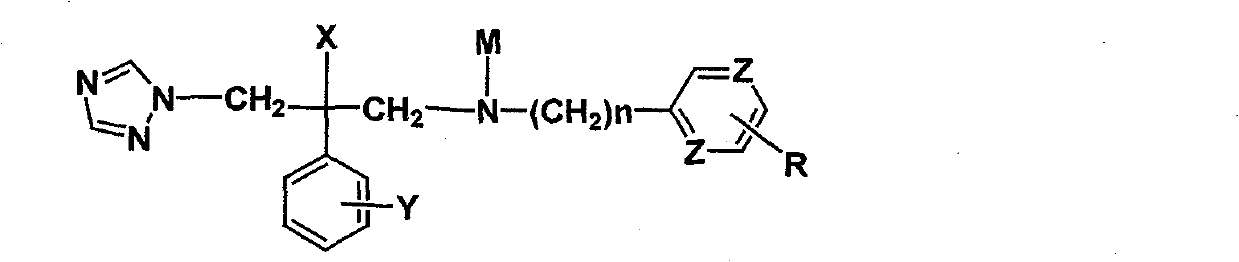
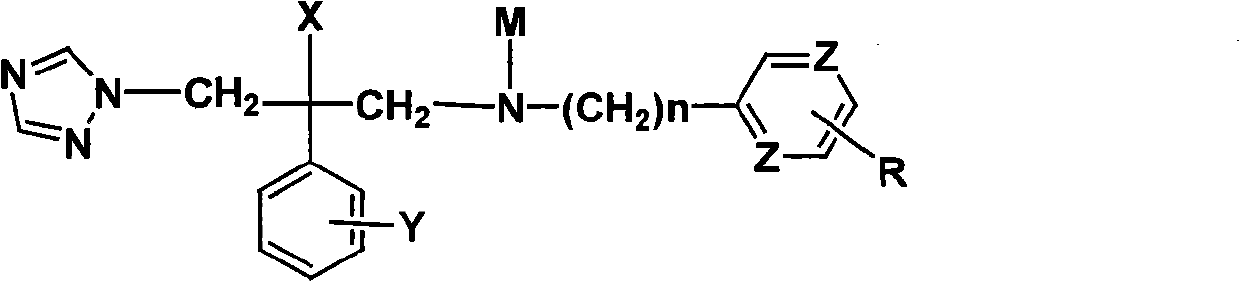
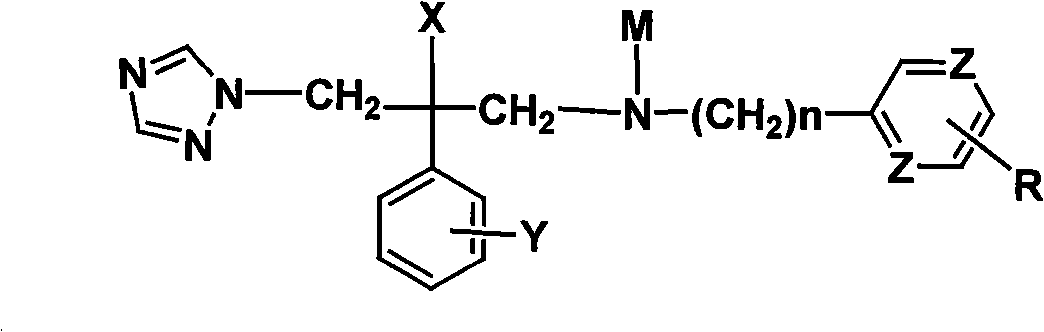
Novel triazole antifungal compound and salt thereof
A kind of triazole compound, compound technology, application in medicine field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Example 1: Preparation of 2-chloro-2', 4'-difluoroacetophenone (II)
[0093] Put 0.7 mol of anhydrous aluminum oxide and 0.67 mol of m-difluorobenzene in a three-necked flask, stir at room temperature, slowly add 0.67 mol of chloroacetyl chloride dropwise, continue stirring at room temperature for 30 min after the dropwise addition, and slowly heat up to 50°C, continue to stir at this temperature for 5 hours, pour the reaction solution into ice water as usual, precipitate crystals, and filter to obtain a solid; the filtrate, after recovering the solvent, obtains a solid, and recrystallizes the obtained solid with methanol to obtain 2-chloro - 107 g of 2',4'-difluoroacetophenone (II). The melting point is 46-47°C.
Embodiment 2
[0094] Example 2: Preparation of 2-(1H-1,2,4-triazol-1-yl)-2',4'-difluoroacetophenone (III)
[0095] Add 0.4mol of triazole, 0.4g of TEBA, and 41.56g of anhydrous potassium carbonate into 180ml of dichloromethane to obtain a suspension; dissolve 38.2g of 2-chloro-2',4'-difluoroacetophenone (II) in In 60ml of dichloromethane, add it dropwise to the above 180ml suspension under ice-bath conditions, drop it for about 1.5h, react at 0-5°C for 5h after dropping, and react at room temperature for 24h. Then filter, wash the filter cake several times with dichloromethane, collect the filtrate, wash the filtrate 3 times with water, dry, evaporate to dryness under reduced pressure, dissolve the residue in anhydrous ethyl acetate, stir and add concentrated nitric acid dropwise to a yellow solid Filter until no more precipitation occurs, the filter cake is washed several times with a small amount of ethyl acetate, and dried to obtain a crude product, which is recrystallized with n-hexane:...
Embodiment 3
[0096] Example 3: Preparation of 1-[2-(2,4-difluorophenyl)-2,3-epoxypropyl]-1H-1,2,4-triazole mesylate (IV)
[0097] Take 0.12mol of 2-(1H-1,2,4-triazol-1-yl)-2′,4′-difluoroacetophenone (III), 0.12mol of trimethyl iodine oxysulfide, trimethyldeca Put 1.6g of hexaalkylammonium bromide into a three-necked bottle, add 180ml of toluene and 250ml of 20% sodium hydroxide solution, heat at 60°C for 3 hours, separate the toluene layer after the reaction, and extract the water layer with toluene for 2 The second time, combine the toluene layers, wash with water until neutral, recover most of the toluene, dilute the residual liquid with ethyl acetate, add 2ml of ethyl acetate containing 8.4g of methanesulfonic acid dropwise at 0°C, precipitate a pale yellow solid, filter , recrystallized from methanol as usual to obtain compound (IV), with a melting point of 128-129°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com