Polymer micelle medicine carrying system using amino acid as stabilizing agent
A technology of polymer glue and amino acid, which can be used in cardiovascular system diseases, antipyretics, anti-inflammatory agents, etc., and can solve problems that have not been seen before
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1 Synthesis of amphiphilic block copolymer mPEG-PDLLA
[0074] Weigh 16 g of methyl polyethylene glycol and 24 g of lactide, place them in a closed reactor, add 50 mg of stannous octoate, and heat up to 120-140°C under nitrogen flow to melt the solid, and raise the temperature to 150-180 React at °C for 6 hours. After cooling, a white solid crude product was obtained. After the crude product is dissolved in 1ml of dichloromethane, it is added to 100ml of ether under stirring, filtered, washed with ether three times, and the product is dried under vacuum for 24 hours.
[0075] The NMR (D-chloroform as solvent) spectrum of the product confirmed the mass ratio and molecular weight of methyl polyethylene glycol to polylactic acid in the polymer with the peak area ratio of 5.2 ppm (PLA) and 3.6 ppm (PEG).
Embodiment 2
[0076] Example 2 Preparation of docetaxel polymer micelles
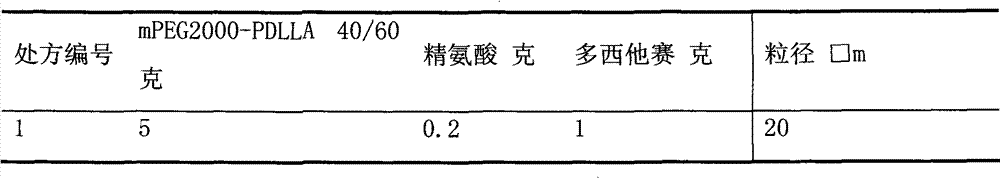
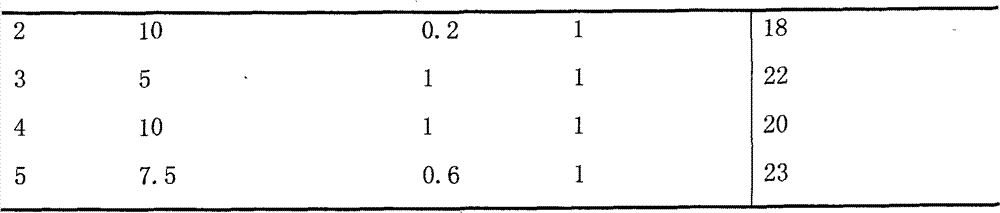
[0077] Pipette 10ml 50mg / ml polymer acetonitrile solution into the eggplant-shaped bottle, add 10ml 5mg / ml docetaxel acetonitrile solution, place the eggplant-shaped bottle on the rotary evaporator, reduce pressure (-0.1MPa), and water bath temperature 50 ℃, rotating speed 20 rpm, rotary evaporation for 30 minutes, add 50ml of 0.2mg / ml arginine aqueous solution and spin for 1 minute, remove the eggplant-shaped bottle, vortex on the vortexer for 1 minute, the resulting solution is filtered through 0.22μm Membrane filtration, take the filtrate into a 10ml vial, 2ml per bottle, immediately freeze-dry to obtain a white solid.
Embodiment 3
[0078] Example 3 Preparation and stability investigation of paclitaxel polymer micelles
[0079] Pipette 6ml 50mg / ml polymer acetonitrile solution into the eggplant-shaped bottle, add 6ml 10mg / ml paclitaxel acetonitrile solution, place the eggplant-shaped bottle on the rotary evaporator, reduce pressure (-0.1MPa), water bath temperature 50℃, rotation speed 20 rpm, rotary evaporation for 30 minutes, add 25ml of 0.2mg / ml aspartic acid aqueous solution and spin for 1 minute, remove the eggplant-shaped bottle, vortex on a vortexer for 1 minute, and filter the resulting solution through a 0.22μm filter membrane , Packed in 10ml vials, 2.5ml per bottle, immediately freeze-dried to obtain a white solid. The solids are mixed with 0.9% sodium chloride injection or 5 ml 5% glucose injection to prepare a clear transparent slightly blue opalescent paclitaxel polymer micelle solution. The resulting micellar solution was kept at 25 degrees Celsius for 24 hours. The results of visual inspectio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com