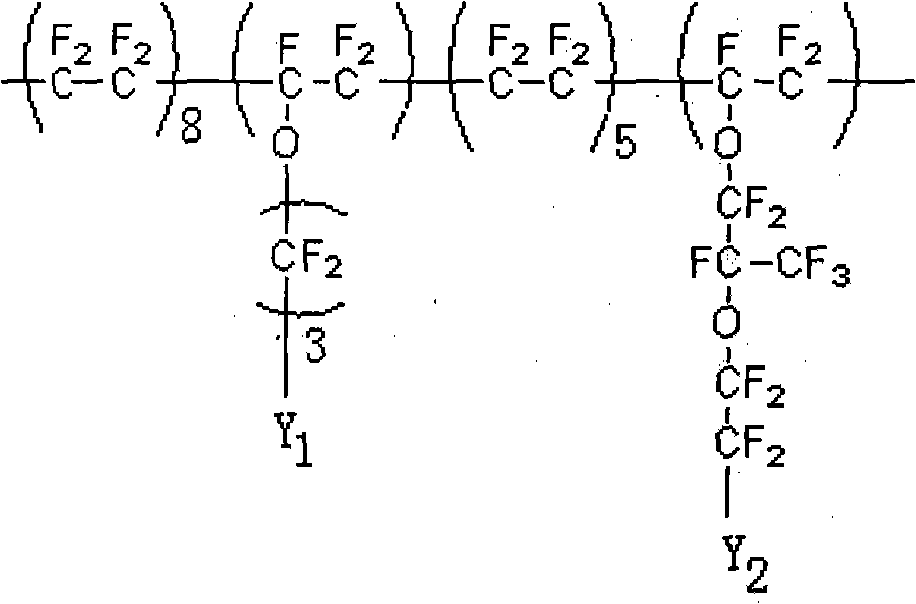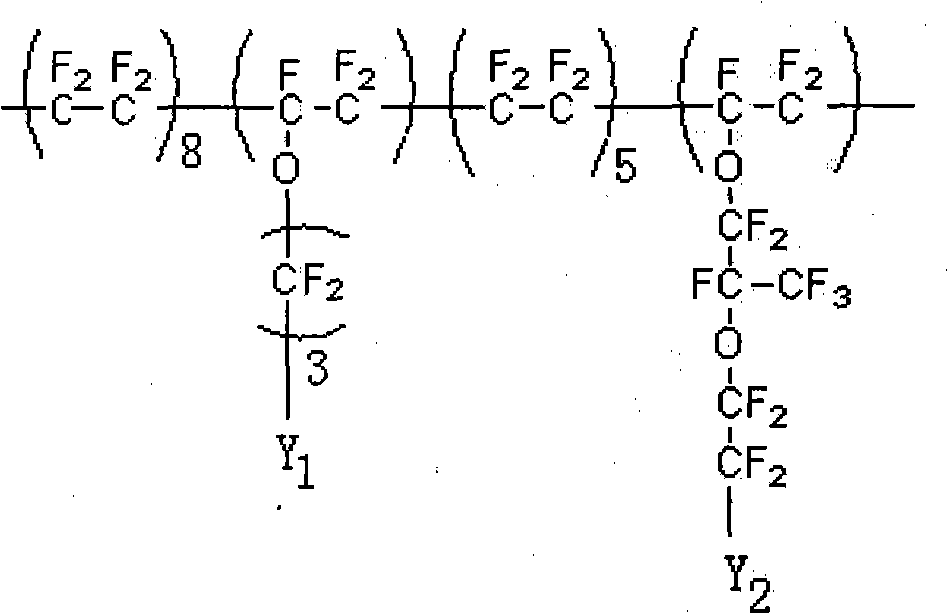Inorganic metal ion mixing with fluorine proton exchange membrane and preparing method thereof
A proton exchange membrane and inorganic metal technology, applied in the field of inorganic metal ion doped fluorine-containing proton exchange membrane and its preparation, can solve the problem of fuel cell durability and service life reduction, chemical deterioration of proton exchange membrane, fuel cell efficiency decline, etc. problems, to achieve the effect of increased proton conductivity, excellent corrosion resistance, and improved utilization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Acid etching and purification of ion-conducting ceramic materials
[0040] Take 2.55g of β-Al 2 o 3Ion-conductive ceramic ultrafine powder, particle size 10-50nm, dried in a vacuum oven at 80°C for 24 hours, added to 100ml of 1.5mol / L HCl after cooling, and ultrasonically treated at 50-70°C for 60 minutes, The precipitate was filtered, washed, and added to a mixed solution of 50 ml of absolute ethanol and water, the volume ratio of absolute ethanol and water was 2:1, ultrasonically treated for 20 minutes, and then the resulting precipitate was dried in a drying oven at 140°C After 10-30 minutes, cool at room temperature to obtain pure β-Al whose surface has been acid-etched. 2 o 3 Ionic conductive ceramic ultrafine powder.
[0041] (2) Surface modification of ion-conducting ceramic materials with thermoplastic resins
[0042] 42.5g polybenzimidazole resin is dissolved in dimethyl sulfoxide, and preparation concentration is the polybenzimidazole resin solution o...
Embodiment 2
[0049] Take 2.55g of SnO 2 Ion-conductive ceramic powder with a particle size of 50-100 nm was placed in a vacuum oven at 80°C for 24 hours and dried for 24 hours. After cooling, 100 ml of 1.5 mol / L HCl was added and ultrasonically treated at 60°C for 40 minutes. The precipitate was filtered, washed and added to a mixture solution of concentrated hydrochloric acid and concentrated nitric acid with a volume ratio of 3:1, treated at room temperature for 10 minutes, filtered and dried to obtain acid-etched pure SnO 2 Ionic conductive ceramic powder.
[0050] 42.5g polyimide resin is dissolved in N-methylpyrrolidone, and preparation concentration is the polyimide resin solution of 8wt%; Get 2.35g above-mentioned SnO through acid etching treatment 2 Ion-conductive ceramic ultrafine powder is dispersed in a mixed solution of 200 ml of anhydrous methanol and water, the volume ratio of anhydrous methanol and water is 2:1, and the obtained dispersion is ultrasonically pulverized and t...
Embodiment 3
[0055] Take 2.55g of mordenite / SnO 2 The composite material, with a particle size of 50-100 nm, was dried in a vacuum oven at 80° C. for 24 hours, and then acid-etched and purified according to the method of Example 1;
[0056] 25.5g polyvinylidene chloride resin is dissolved in N, N-dimethylacetamide, the preparation concentration is the polyvinylidene chloride resin solution of 10wt%; Get 2.35g above-mentioned mordenite / SnO through acid etching treatment 2 The composite material is dispersed in a mixed solution of 200 milliliters of absolute ethanol and water, the volume ratio of absolute ethanol and water is 2: 1, and the obtained dispersion is added to the obtained polyvinylidene chloride resin solution after ultrasonic pulverization; fully Stir and disperse evenly to obtain polyvinylidene chloride resin-modified mordenite / SnO 2 of the dispersion.
[0057] Take 0.5g of cerium nitrate and dissolve it in ethanol-water mixed solution, and slowly add it dropwise to the dispe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com