Real-time feature extraction method for analysis of complex ingredient of traditional Chinese medicine
A component analysis and real-time feature technology, applied in medical preparations containing active ingredients, analytical materials, pharmaceutical formulations, etc., it can solve the problems that the parameters have no actual physical meaning and are difficult for ordinary users to understand, so as to achieve fewer parameters and improve efficiency. , optimize the simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
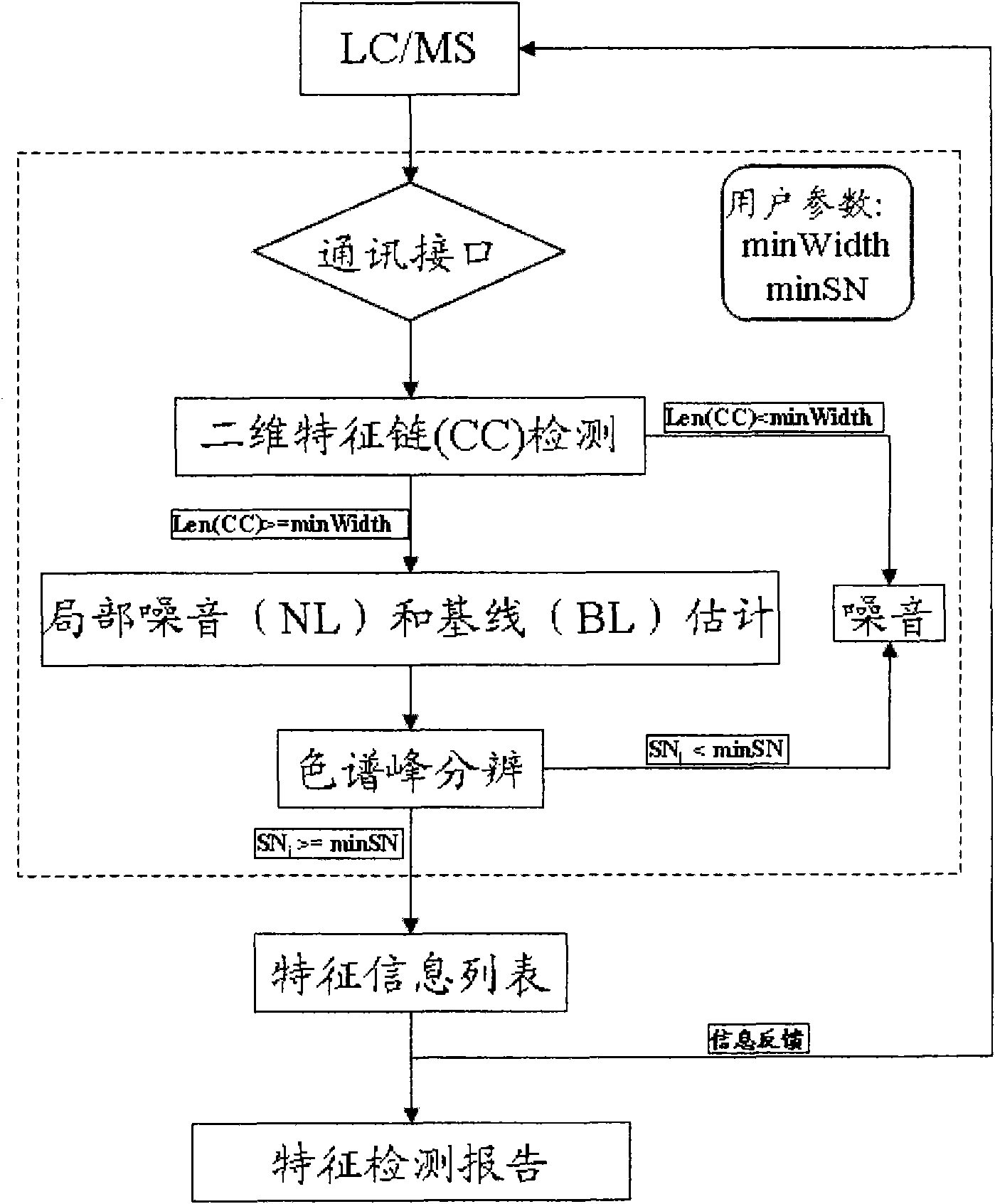
[0036] Embodiment 1 A kind of real-time feature extraction method for complex component analysis of traditional Chinese medicine of the present invention
[0037] 1. Communication module: MS_Communication (acq_mode, cur_ms_data)
[0038] This function is responsible for communicating with the mass spectrometer. If the acquisition mode (acq_mode) is profile, when the current data is obtained from the mass spectrometer, it will be converted into centroid format using the watershed algorithm, and returned through the cur_ms_data parameter; if the acquisition mode is centroid, then Return the data directly. The parameter cur_ms_data is two-dimensional data containing the mass-to-charge ratio and its corresponding intensity.
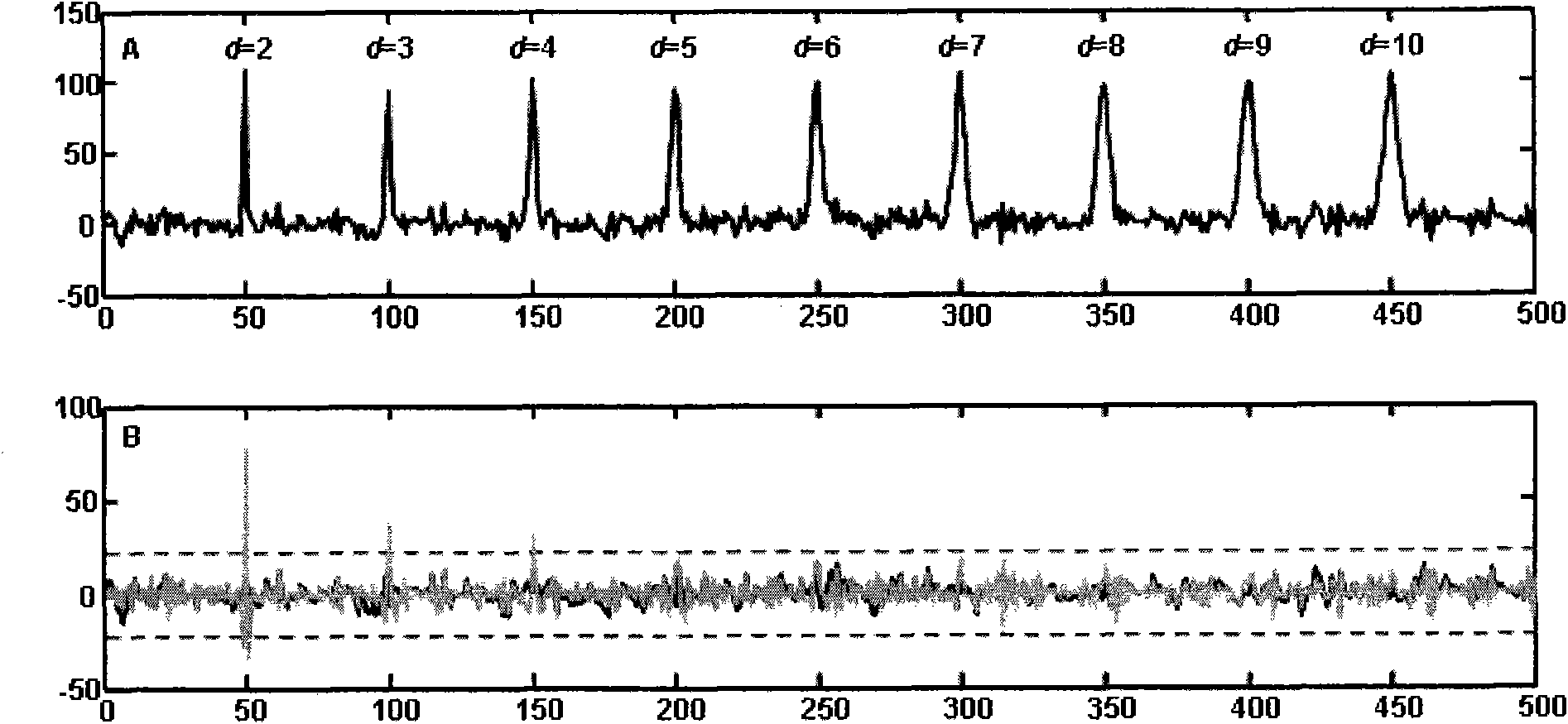
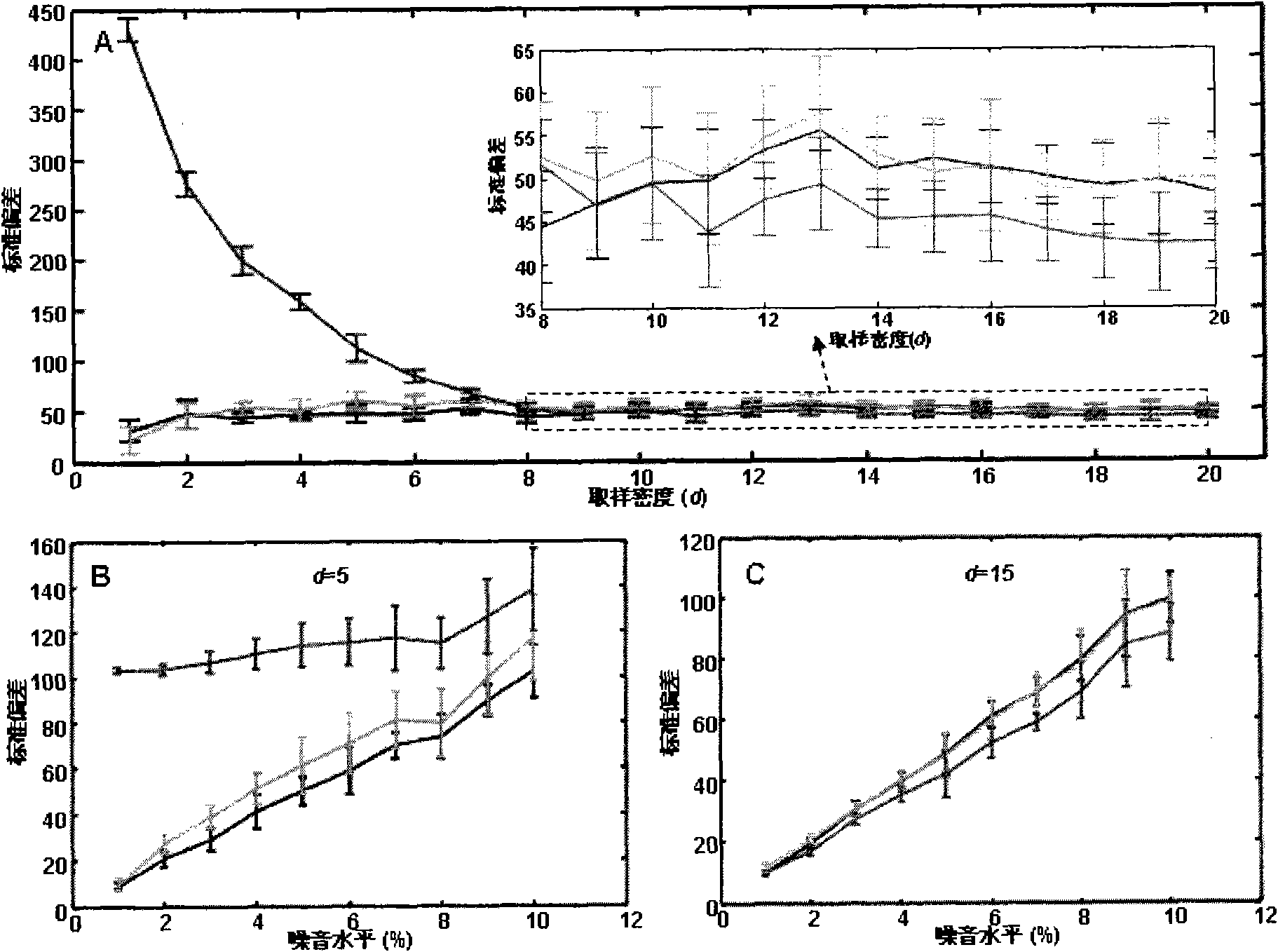
[0039] 2. Two-dimensional feature chain detection: BNN (minWidth, CC)
[0040] In the BNN module, by calling MS_Communication, the currently collected mass spectrum data can be obtained and assigned to the mass-to-charge ratio array MZ and the intensity arr...
Embodiment 2
[0057] Example 2 Analysis of Complex Components in Weifuchun Tablets
[0058] A. Preparation of total extract of Weifuchun Tablets
[0059] Take 20 Weifuchun Tablets, remove the film coating, and grind into fine powder. Accurately weigh 0.5g and place it in a 50mL Erlenmeyer flask with a stopper, accurately add 10mL of methanol, and conduct ultrasonic extraction for 45 minutes. After the extraction, take out the Erlenmeyer flask, and make up the weight with methanol solution after cooling. The extract was shaken up and centrifuged at 12000 rpm for 15 min, and the supernatant was filtered through a 0.45 μm filter membrane for HPLC analysis.
[0060] B. Chromatographic and mass spectrometric conditions for LC-MS analysis
[0061] The liquid phase is an Agilent1100 high performance liquid chromatograph (Agilent, USA), equipped with a binary gradient pump, a DAD ultraviolet detector, a column thermostat, and an autosampler. Column: ZORBAX SB-C 18 Chromatographic column (4.6mm...
Embodiment 3
[0065] Example 3 Shuangdan Granule Complex Component Analysis
[0066] A. Preparation of Shuangdan Granule Samples
[0067]Precisely weigh 0.05 g of Shuangdan granules after fine grinding (Shandong Kongshengtang Pharmaceutical Co., Ltd., batch number: 040201, 031001), add Wahaha purified water 1mL, ultrasonically extract for 20min, then centrifuge at 10000rpm for 10min, take 0.5mL of the supernatant, wash with methanol - Dilute 1-fold with water-formic acid (50:50:1).
[0068] B. Chromatographic and mass spectrometric conditions for LC-MS analysis
[0069] Agilent 1100 liquid chromatography system, including binary high pressure pump, automatic sampler, column thermostat and DAD detector. Chromatographic column: Agilent SB-C18 (2.1×250mm, 3.5m). Mobile phase: 0.1% formic acid acetonitrile (A)-0.1% formic acid water (B), phase A rises linearly from 10% to 20% in 0-5min, 40% in 5-7min, and 95% in 7-20min %; flow rate 0.3mL / min, column temperature 35°C. All analyzed samples ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com