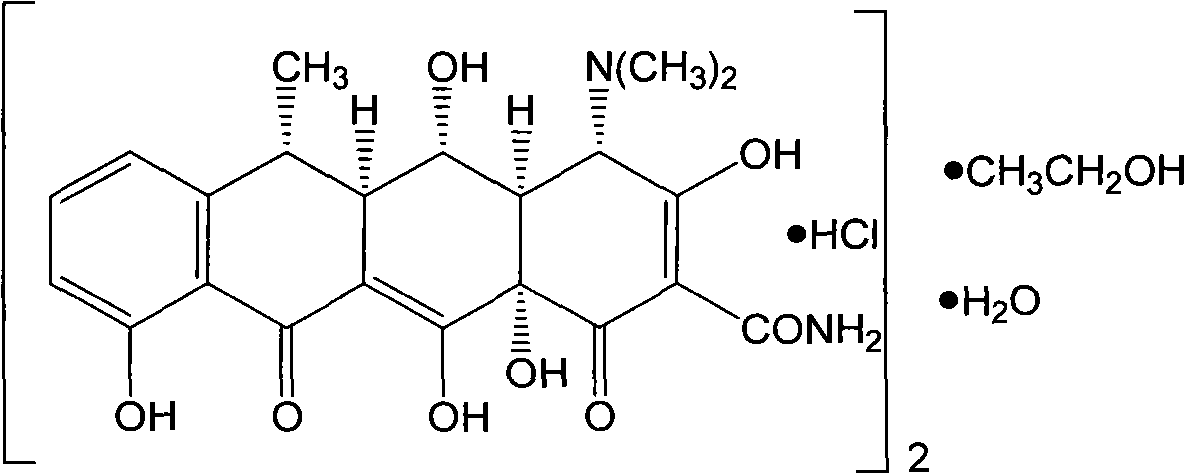
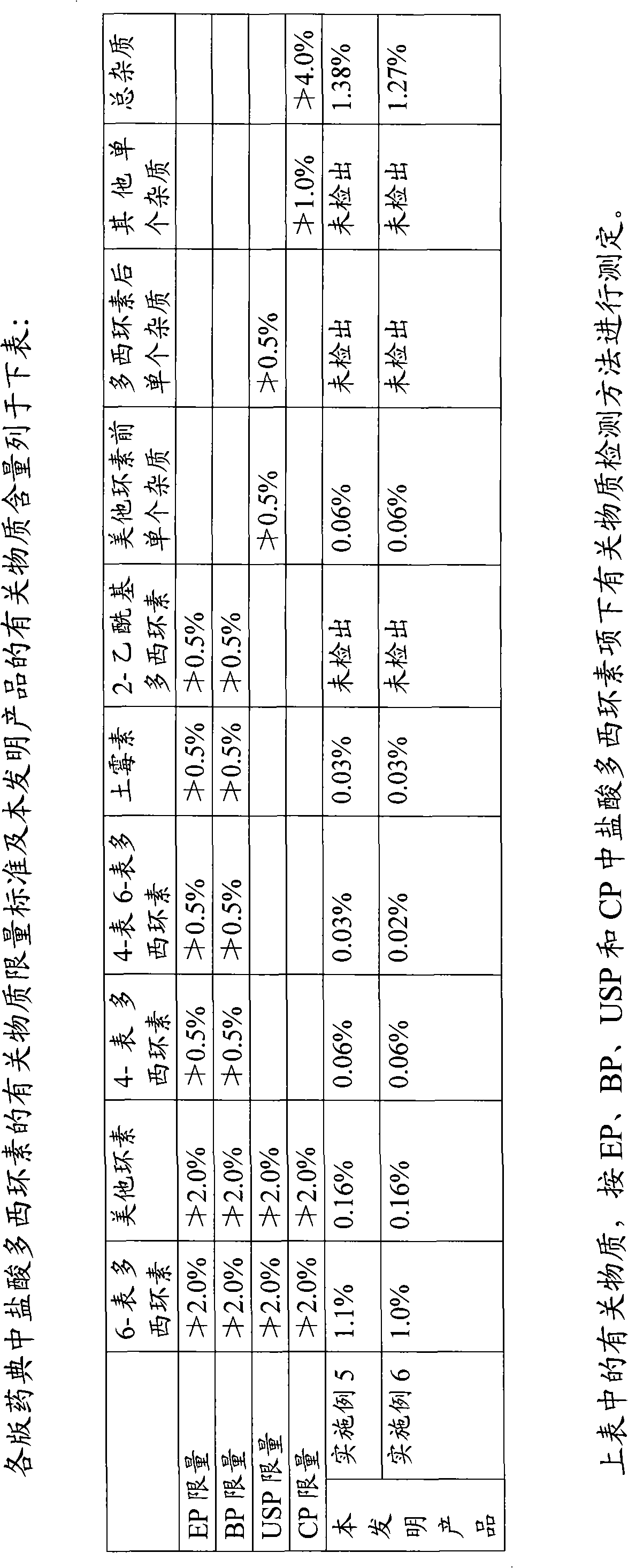
Preparation process of doxycycline hydrochloride
A technology of doxycycline hydrochloride and preparation process, applied in the field of preparation of doxycycline hydrochloride, can solve the problems of poor stereospecificity, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 60ml of 60% ethanol to the hydrogenation tank, add metronidazole 0.0062g, 4-picoline 0.018g and 2,4-dihydroxyquinoline 0.063g solution in 5ml methanol, 5% palladium / carbon 0.8 g, stirred for 20 minutes, added 10 g of 11α-chloro-6-methine oxytetracycline p-toluenesulfonate, stirred for 30 minutes, and sealed. Vacuumize and change nitrogen for 3 times, then change hydrogen for 3 times, heat up, and react with hydrogen at 65°C, keeping the pressure at 6.5kg / cm 2 , After the completion of the reaction was confirmed by thin-plate chromatography, the reaction was stopped. Filter, wash with ethanol and water successively, combine the filter and washing liquid, add 5-sulfosalicylic acid, stir at 40°C for 1 hour, stand at 20°C for 6 hours, filter, wash with ethanol, and drain to obtain α-6-deoxy Oxytetracycline 5-sulfosalicylate 8.6g (dried).
Embodiment 2
[0033] Put 60ml of 60% ethanol into the hydrogenation kettle, add metronidazole 0.0057g, 4-picoline 0.016g and 2,4-dihydroxyquinoline 0.058g solution in 5ml methanol, 5% palladium / carbon 0.8 g, stirred for 20 minutes, added 10 g of 11α-chloro-6-methine oxytetracycline p-toluenesulfonate, stirred for 30 minutes, and sealed. Vacuumize and change nitrogen for 3 times, then change hydrogen for 3 times, heat up, and react with hydrogen at 65°C, keeping the pressure at 5.5kg / cm 2, After the completion of the reaction was confirmed by thin-plate chromatography, the reaction was stopped. Filter, wash with ethanol and water successively, combine the filter and washing liquid, add 5-sulfosalicylic acid, stir at 40°C for 1 hour, stand at 20°C for 6 hours, filter, wash with ethanol, and drain to obtain α-6-deoxy Oxytetracycline 5-sulfosalicylate 9.3g (dried).
Embodiment 3
[0035] Put 30ml of 60% ethanol and 10g of α-6-deoxyoxytetracycline 5-sulfosalicylate into the reactor successively, stir, add ammonia water dropwise at 10°C until the pH is 5.9, continue stirring for 15 minutes, and keep the pH at 5.9 No change, stop dripping ammonia water. Raise the temperature to 35°C, stir for 30 minutes, keep warm for 4 hours, filter, wash with 60% ethanol, and drain to obtain 6.0 g of α-6-deoxyoxytetracycline base (dried).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com