Malignant lymphoma radioactive target molecule imaging agent and/or target therapeutic agent
A targeted therapeutic agent and targeted molecular technology, which is applied in the direction of radioactive carriers, antineoplastic drugs, drug combinations, etc. In order to achieve the effects of enriching theory and clinical practice, improving penetrating power, and convenient and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Preparation of Small Molecule Fusion Peptide
[0049]According to Qiu Xiaoqing et al. in the literature (Xiao-Qing Qiu, He Wang, Bei Cai, Lan-Lan Wang&Shi-Tao Yue. Small antibody mimetics comprising two complementarity-determining regions and a framework region for tumor targeting. NATURE BIOTECHNOLOGY, 2007: 25(8) : 921-928) prepared small molecule fusion peptide, or synthesized by Xi'an Lianmei Biological Products Co., Ltd. according to the amino acid sequence disclosed in SEQ ID NO.1-SEQ ID NO.5.
Embodiment 2
[0050] Embodiment 2 prepares imaging agent of the present invention
[0051] 1.1 Materials
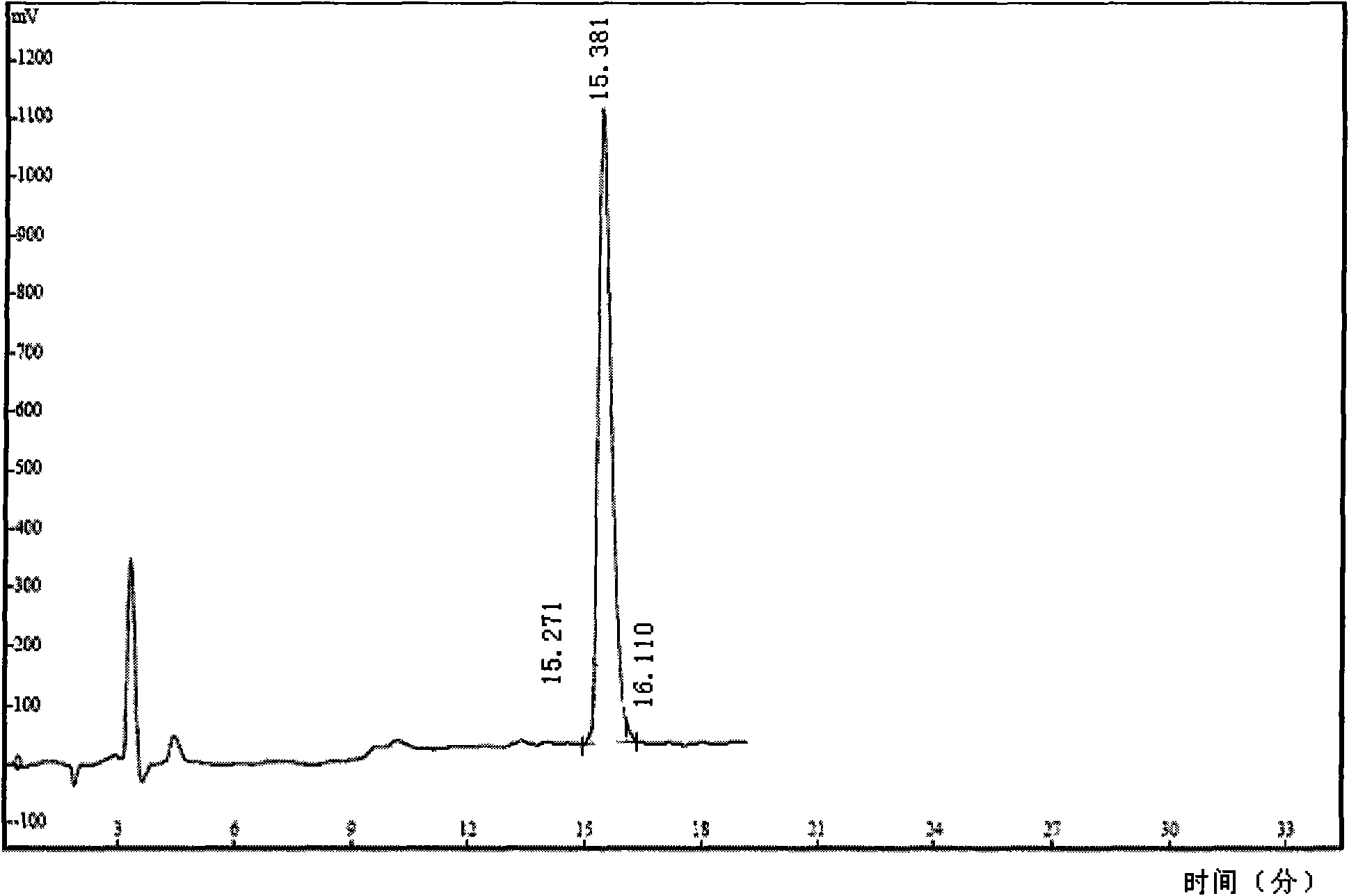
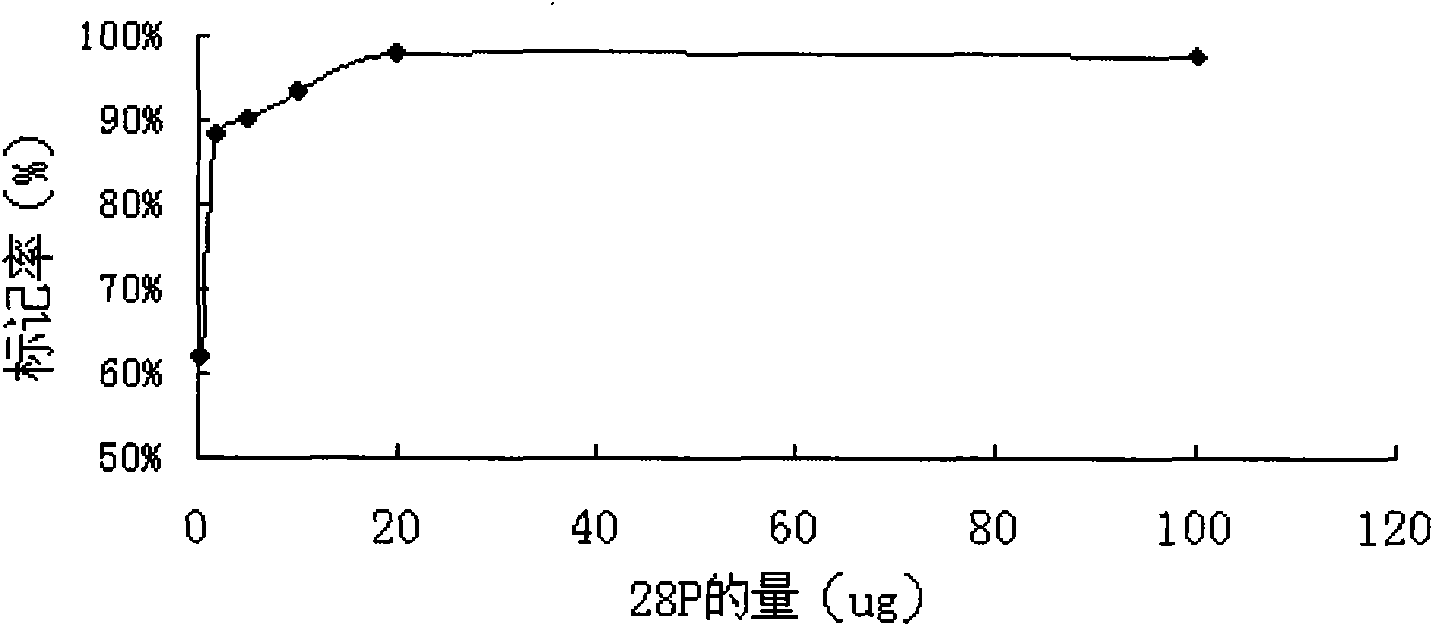
[0052] 28P (SEQ ID NO.1) was synthesized by Xi'an Lianmei Biological Products Co., Ltd. with a purity of >95% (see figure 1 ).
[0053] Use 24 BALB / c mice, body weight about 20g, male or female, be divided into 7 groups, 5 in each group (provided by Experimental Animal Center of Sichuan University); 125 I / Na 131 I is produced by Chengdu Zhonghe Qualcomm Isotope Co., Ltd. (no carrier, no reducing agent, radiochemical purity ≥ 96%, pH value 7.0~8.0, gamma impurity < 0.1%); N-bromosuccinimide is a sigma product ( USA), HAS (Shanghai), analytically pure; acetone (Chengdu Xinchuan Chemical Reagent Co., Ltd.), gamma counter (state-owned 262 factory), ESJ120-4 electronic analytical balance (Shenyang), vortex mixer XW-80A ( Shanghai Medical University).
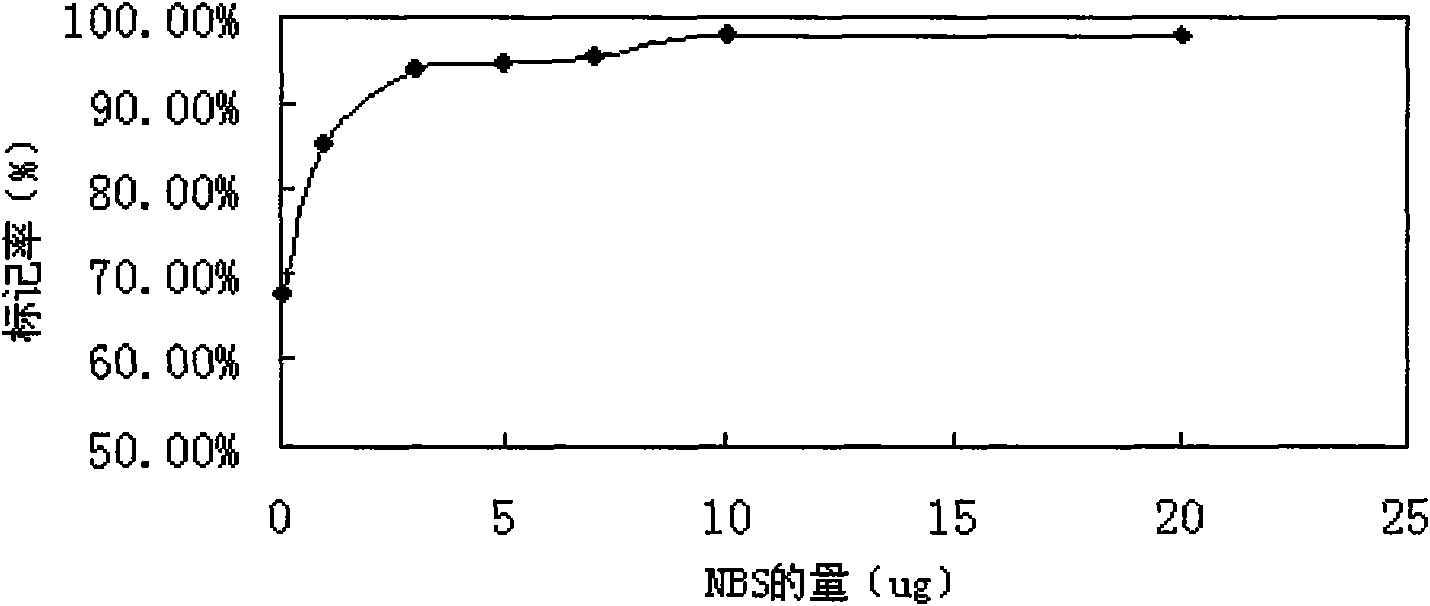
[0054] 2.1 28P 125 I labeling and determination of optimal labeling conditions
[0055] N-bromosuccinimide (NBS) was used as an oxida...
Embodiment 3
[0094] Example 3 Detection of distribution of iodine-125 labeled 28P in normal mice
[0095] Preparation of iodine-125 labeled 28P: take 10 μl Na 125 I (about 1.5mCi), then add 10μl (1μg / μl) NBS solution, then add pre-packed 1μg / μl 28P, and finally add 100μl 0.05mol / L PBS buffer (pH7.2~7.4) and mix well. Shake and mix slowly, react for 3 minutes, add 50 μl of 2% HSA solution to terminate the reaction for 30 seconds, and labeling is completed. Paper chromatography measures the labeling rate, the stationary phase is Xinhua No. I paper, and the developer is acetone: normal saline=1: 1 (volume ratio), 125 Rf of I-28P=0.4~0.6, free 125 The Rf of I was 0.8-1.0, and the labeling rate was identified by TLC scanner. acquired 131 The radiochemical purity of I-28P is 96.8%.
[0096] 125 Biodistribution of I-28P marker in normal mice Thirty-five healthy Kunming mice were selected and randomly divided into 7 groups with 5 mice in each group. Each mouse was injected via the tail vein...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com