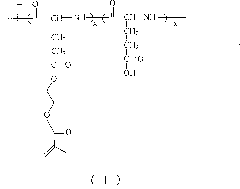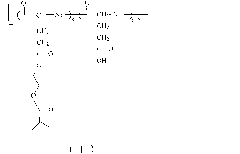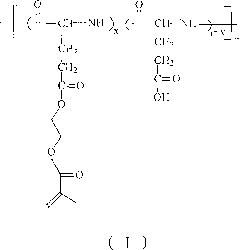Poly (L-glutamic acid)/polyacrylic acid hydrogel and preparation method thereof
A technology of polyacrylic acid and glutamic acid, applied in the field of hydrogels, can solve the problems of long release time, increased metabolic burden, inappropriateness, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] Another embodiment of the present invention discloses a preparation method of poly(L-glutamic acid) / polyacrylic acid hydrogel, including:
[0033] a) Adjust the pH value of the buffer solution of acrylic acid to 8-9 to obtain the first reaction solution;
[0034] b) adding a buffer solution of poly(L-glutamic acid-g-hydroxyethyl methacrylate) to the first reaction solution to obtain a second reaction solution;
[0035] c) adding an initiator to the second reaction solution to obtain a third reaction solution, and heating or irradiating the third reaction solution to obtain a reaction product;
[0036] d). Soak the reaction product in deionized water to obtain poly(L-glutamic acid) / polyacrylic acid hydrogel.
[0037] According to the present invention, the purpose of adjusting the pH value of the buffer solution of acrylic acid to 8-9 is to make the first reaction solution weakly alkaline, because poly(L-glutamic acid-g-hydroxyethyl methacrylate) is insoluble The acidic solution ...
Embodiment 1
[0053] 1. Take 0.005mol of BLG-NCA monomer into a dry round bottom flask, add 300mL of anhydrous dioxane to the round bottom flask, the BLG-NCA monomer will dissolve, add while stirring the solution in the round bottom flask 0.002mol of triethylamine, then the solution was stirred and reacted at room temperature for 72h, and finally the solution was settled with 3000mL ethanol, filtered, washed with ethanol three times, and dried under vacuum at room temperature for 24h to obtain poly(γ-benzyl-L-gu Amino acid ester).
[0054] 2. Take 5g of the poly(γ-benzyl-L-glutamate) prepared in step 1, dissolve it with 50mL of dichloroacetic acid and add 30mL of hydrogen bromide / glacial acetic acid with a mass content of hydrogen bromide of 33% while stirring. Then, the solution was stirred and reacted at 30° C. for 1.5 hours, and finally settled with 500 mL of acetone, filtered, washed with acetone three times, and vacuum dried at room temperature for 24 hours to obtain poly(L-glutamic acid)...
Embodiment 2
[0056] Take 1g of poly(L-glutamic acid) prepared in Example 1 and mix with 0.1g of hydroxyethyl methacrylate monomer, and dissolve in 30mL DMSO, dissolve all the solids to obtain solution a; then add 0.20g of N, N'-cyclohexylcarbodiimide and 0.10g 4-dimethylaminopyridine were dissolved in 1.5mL DMSO to obtain solution b; pour the above solution b into solution a, react at room temperature for 72 hours, and then filter out the resulting precipitate The filtrate was settled with 400 mL of ether, filtered, washed with ether for three times, dried in vacuum at room temperature for 24 hours, and then dried in vacuum at 40° C. for 24 hours to obtain a polymer with the structure of Formula II.
[0057]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com