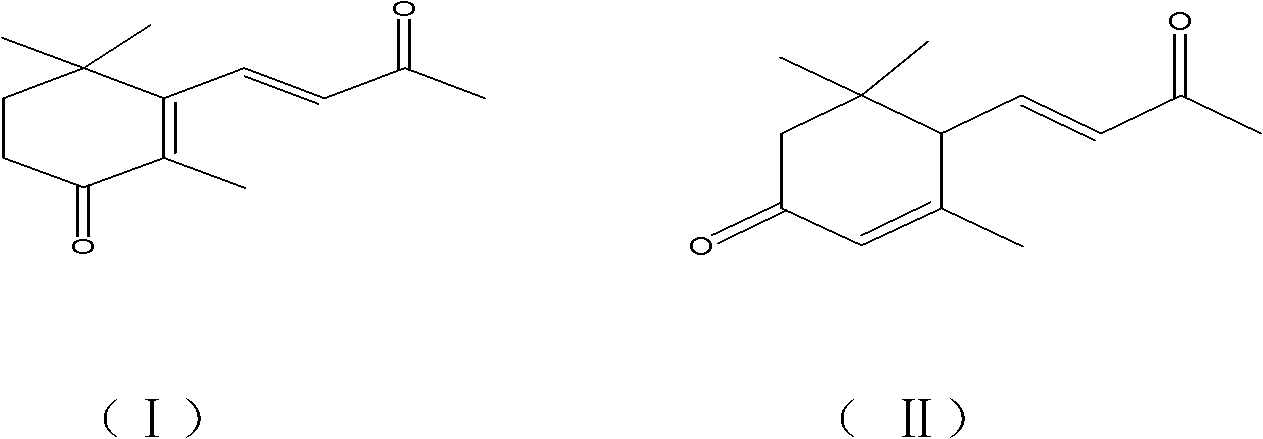
Method for synthesizing oxo-alpha-ionone or oxo-beta-ionone
A technology of ionone and oxo, which is applied in the field of oxo α or β-ionone, can solve the problems of less than 40% yield, unsatisfactory yield, lengthy synthesis process, etc., and achieves shortened reaction time and cost. Low, the effect of improving the reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: Synthesis of 4-oxo-β-ionone
[0017] 19.2g of β-ionone, 13.5g of sodium bromate, 40g of 7% sulfuric acid solution, and 60ml of tetrahydrofuran were reacted at a controlled temperature of about 45°C. After 2.0 hours, samples were taken for GC-MS detection. The conversion of raw materials was basically complete, and the reaction was stopped. The GC-MS detection results showed that the chromatographic peak area ratio of 4-oxo-β-ionone was 91.7%, and the chromatographic peak area ratio of 5,6-epoxy-β-ionone was 7.5%. Separate the liquid, wash with water, remove the salt solution, extract the aqueous solution with 2*20ml of ether, combine the organic phases, rotary evaporate, and evaporate the solvent to dryness to obtain (yield: 67%) 4-oxo-β-ionone.
[0018] The isolated 4-oxo-β-ionone is a colorless crystal with a melting point of 50°C to 52°C. Through GC-MS analysis, its molecular ion peak is 206 (molecular ion peak M + , abundance 72%), 163 (M + CH 3 CO...
Embodiment 2
[0020] Embodiment 2: Synthesis of 4-oxo-β-ionone
[0021] 19.2g of β-ionone, 13.5g of sodium bromate, 40g of 12% sulfuric acid solution, and 60ml of tetrahydrofuran were reacted at a controlled temperature of about 45°C. After 1.5 hours, samples were taken for GC-MS detection. The conversion of raw materials was basically complete, and the reaction was stopped. The GC-MS detection results showed that the chromatographic peak area ratio of 4-oxo-β-ionone was 84.5%. 4-Oxo-β-ionone was obtained (yield: 58%).
[0022] Subsequent reaction is the same as in Example 1, below.
Embodiment 3
[0023] Embodiment 3: Synthesis of 4-oxo-β-ionone
[0024] β-ionone 19.2g, sodium bromate 13.5g, 7% sulfuric acid solution 40g, 60ml acetone, control the temperature to react at about 45°C, take a sample after 2.0h for detection by GC-MS, 4-oxo-β-ionone The peak area of the chromatographic peak reaches 78%. 4-Oxo-β-ionone was obtained (yield: 47%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com