Nicotinic acid-contained curcumin ester derivative and preparation method and application thereof
A technology of nicotinic acid curcumin ester and acid curcumin ester, applied in the field of nicotinic acid curcumin ester derivatives and their manufacture, can solve problems such as failure to effectively solve problems, achieve convenient industrial production, excellent blood lipid lowering, less toxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
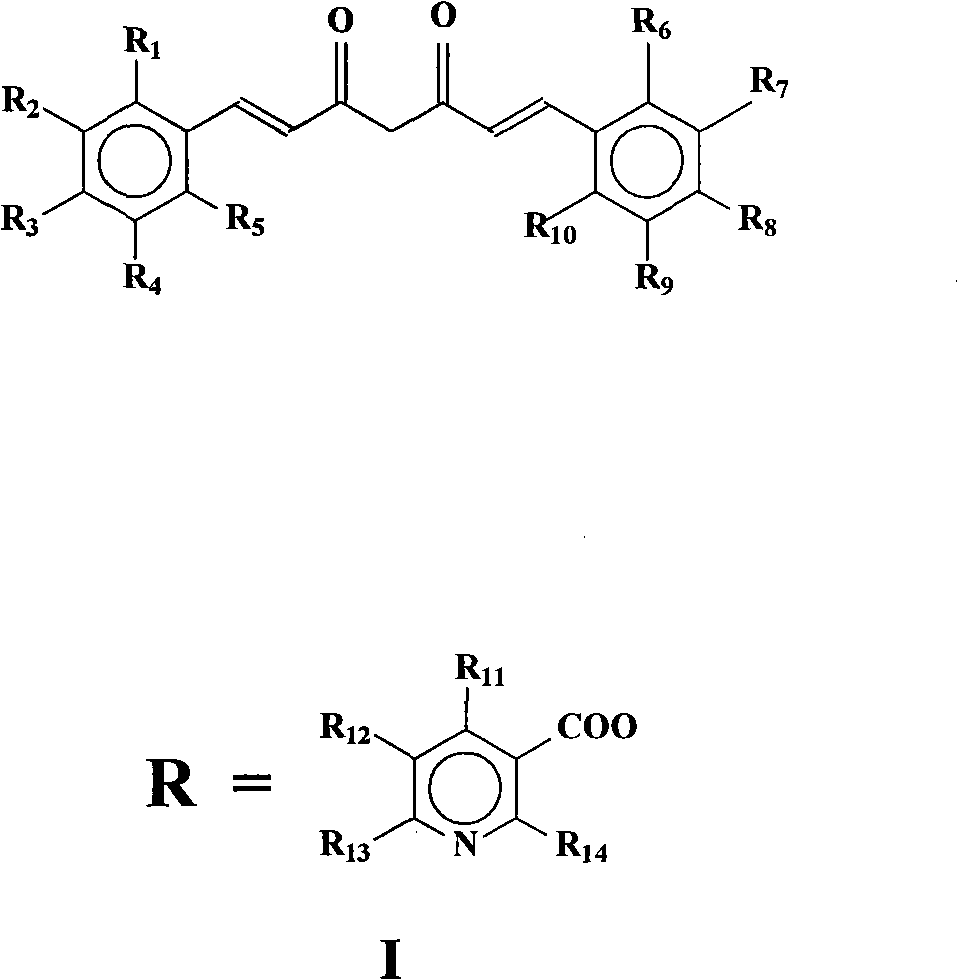
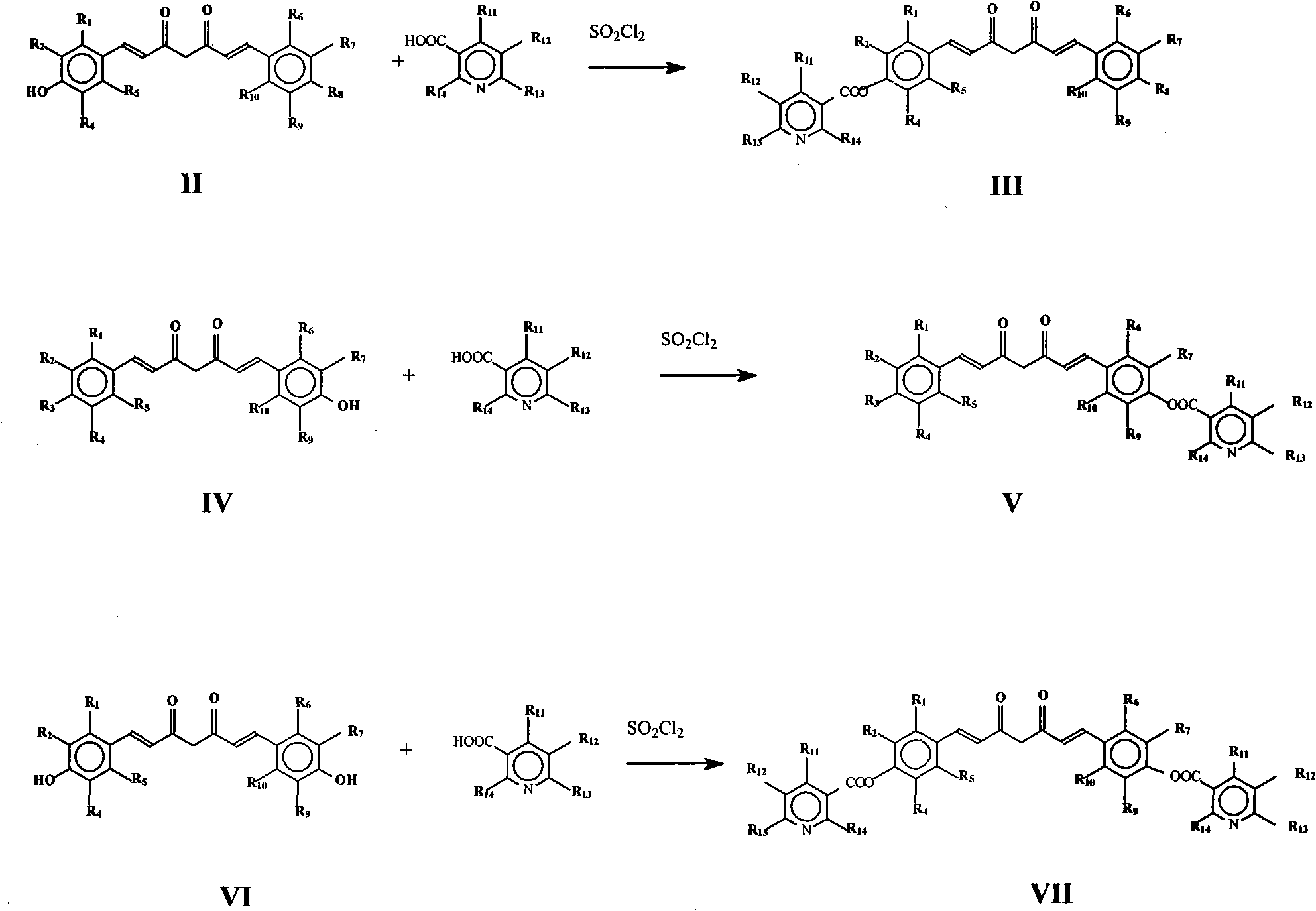
[0055] 4′-Hydroxy-4″-nicotinic acid curcuminate (code 101), 4″-hydroxy-4′-nicotinic acid curcuminate (code 109) and 4′,4″-nicotinic acid curcuminate (code 109) 118) Preparation:
[0056] React 0.01mol curcumin in 10ml thionyl chloride with 0.03mol nicotinic acid at 75°C for 10 hours, pour into an ice-water bath, extract 3 times with ethyl acetate, dry over anhydrous sodium sulfate, and then use ethyl acetate : Petroleum ether=1: 3 column chromatography, obtain target product 4 '-hydroxyl-4 "-nicotinic acid curcuminate (code number 101), 4 "-hydroxyl-4'-nicotinic acid curcuminate (code number 109) and 4′, 4″-curcumin dinicotinate (code 118). Relevant data are as follows:
[0057] 4′-Hydroxy-4″-nicotinic acid curcuminate (code 101): MS (EI, 70ev) m / z: 473; Anal.Calcd.for C 27 h 23 NO 7 : C, 68.49, H, 4.90, N 2.96; Found C, 68.22, H, 4.80, N 2.95.
[0058] 4″-Hydroxy-4′-nicotinic acid curcuminate (code 109): MS (EI, 70ev) m / z: 473; Anal.Calcd.for C 27 h 23 NO 7 : C, 68.49...
Embodiment 2
[0061] The preparation of 4'-methoxy-4 "-nicotinic acid curcuminate (code name 102):
[0062] Dissolve 0.03mol methyl iodide and 0.01mol 4′-hydroxy-4″-nicotinic acid curcuminate (code 101) in 25ml acetone, add 10 grams of catalyst K 2 CO 3 , reacted at 60°C for 4 hours to obtain the target product 4'-methoxy-4"-nicotinic acid curcuminate. The relevant data are as follows:
[0063] MS (EI, 70ev) m / z: 487; Anal. Calcd. for C 28 h 25 NO 7 : C, 68.98, H, 5.17, N 2.87; Found C, 68.93, H, 5.19, N 2.86.
Embodiment 3
[0065] 4'-Ethoxy-4"-nicotinic acid curcuminate (code 103), 4'-propoxy-4"-nicotinic acid curcuminate (code 104), 4'-benzyloxy-4"- Nicotinic acid curcuminate (code 105), 4'-heptyloxy-4"-nicotinic acid curcuminate (code 106), 4'-octyloxy-4"-nicotinic acid curcuminate (code 107), Preparation of 4'-decyloxy-4"-nicotinic acid curcuminate (code number 108):
[0066] Replace methyl iodide with 0.03mol haloalkane according to the operation of Example 2 to obtain compound 4'-ethoxy-4"-nicotinic acid curcuminate (code number 103), 4'-propoxyl-4"-nicotinic acid curcumin Ester (code 104), 4'-benzyloxy-4"-nicotinic acid curcuminate (code 105), 4'-heptyloxy-4"-nicotinic acid curcuminate (code 106), 4'-octyl Oxy-4″-nicotinic acid curcuminate (code 107), 4′-decyloxy-4″-nicotinic acid curcuminate (code 108). The relevant data are as follows:
[0067] 4′-Ethoxy-4″-nicotinic acid curcuminate (code 103): MS (EI, 70ev) m / z: 501; Anal.Calcd.for C 29 h 27 NO 7 : C, 69.45, H, 5.43, N 2.79; Found...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com