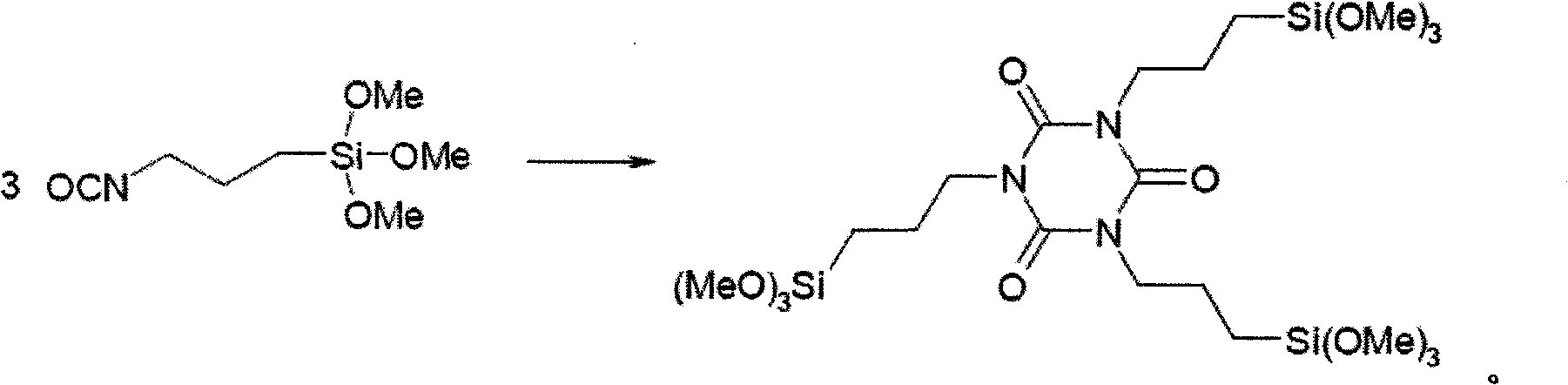
Method for preparing tri(3-trimethoxysilylpropyl) isocyanurate
A technology of trimethoxysilylpropyl and trimethoxysilyl, which is applied in the field of preparation of triisocyanurate, can solve the problems of many side reactions, unsatisfactory, slow reaction speed, etc., so as to save reaction time , Improve production efficiency, improve the effect of reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Put 2050ml of dimethyl sulfoxide, 2052.8g (10mol) of 3-isocyanatopropyltrimethoxysilane (99.0% GC content) and 10.8g (0.2mol) of sodium methylate into the reactor, and pass through the reactor with nitrogen replacement In the air, the temperature was raised to 100°C, and the reaction was maintained for 6 hours, then the temperature was lowered to 20°C, and the insoluble matter was removed by filtration to obtain a filtrate. The filtrate is concentrated at a temperature of 150° C. under a vacuum of 15 Pa until there is no distillate under negative pressure to obtain a reddish-brown viscous liquid 1847g (i.e. crude product, content 85%), the selectivity of the product is 98.5%, and it is divided into 100g / min speed into 0.2m 2 In the molecular still, the temperature of the molecular still is controlled to be 200°C, the vacuum degree is 5Pa, and the light component obtained is 1,3,5-tris[3-(trimethoxysilyl) Propyl]-1,3,5-triazine-2,4,6-trione, weight 1535g, yield 74.78%,...
Embodiment 2
[0027] Put 3075ml of dimethyl sulfoxide, 2052.8g (10mol) of 3-isocyanatopropyltrimethoxysilane (99.0% GC content) and 16.2g (0.3mol) of sodium methoxide into the reactor, and pass through the reactor to replace it with nitrogen In the air, the temperature was raised to 130°C, and the reaction was kept for 4 hours, then the temperature was lowered to 20°C, and the insoluble matter was removed by filtration. The filtrate is concentrated at a temperature of 180° C. under a vacuum of 15 Pa until there is no distillate under negative pressure to obtain 1865 g of a reddish-brown viscous liquid (i.e. crude product, content 83.4%). The selectivity of the product is 97.6%, which is divided into 100 g / min speed into 0.2m 2 In the molecular still, the temperature of the molecular still is controlled to be 200°C, the vacuum degree is 2Pa, and the light component obtained is 1,3,5-tris[3-(trimethoxysilyl) Propyl]-1,3,5-triazine-2,4,6-trione, weight 1570g, yield 76.48%, content (ie purity...
Embodiment 3
[0029] Put 2052ml of N, N-dimethylformamide, 2052.8g (10mol) of 3-isocyanatopropyltrimethoxysilane (GC content 99.0%), 21.6g (0.4mol) of sodium methoxide into the reactor, and feed nitrogen The air in the reactor was replaced, the temperature was raised to 150° C., the temperature was kept for 3 hours, the temperature was lowered to 20° C., and the insoluble matter was removed by filtration. The filtrate is concentrated at a temperature of 150° C. under a vacuum of 15 Pa until no distillate is obtained, and 1901 g of a reddish-brown viscous liquid (i.e. crude product, content 86.1%) is obtained. The selectivity of the product is 99.1%. / min speed into 0.2m 2 In the molecular still, the temperature of the molecular still is controlled to be 230°C, the vacuum degree is 10Pa, and the light component obtained is 1,3,5-tris[3-(trimethoxysilyl) Propyl]-1,3,5-triazine-2,4,6-trione, weight 1601g, yield 77.99%, content (ie purity) 98.05%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com