Method for measuring content of superfine powder preparation in five retentions powder
A technology of ultrafine powder and determination method, which is applied in the direction of medical preparations containing active ingredients, anti-inflammatory agents, measuring devices, etc., and can solve problems such as single quality standards and no simultaneous detection of multiple components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 5
[0018] The content determination of 11 kinds of indicators in the Wujisan superfine powder preparation of embodiment 1
[0019] 1. Chromatographic conditions:
[0020] Chromatographic column: Octadecyl silica gel column ((5.0μm, 250mm×4.6mm)
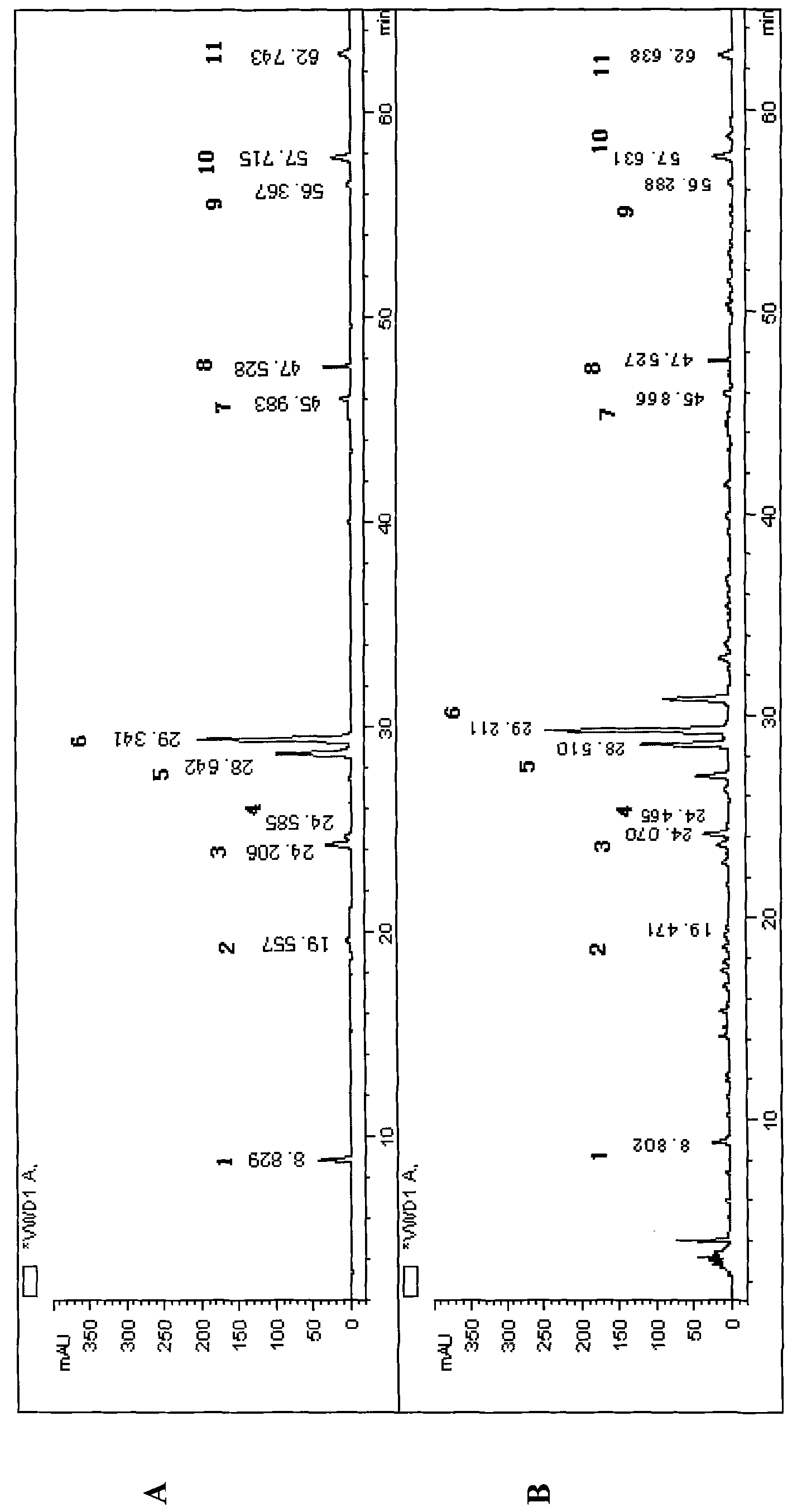
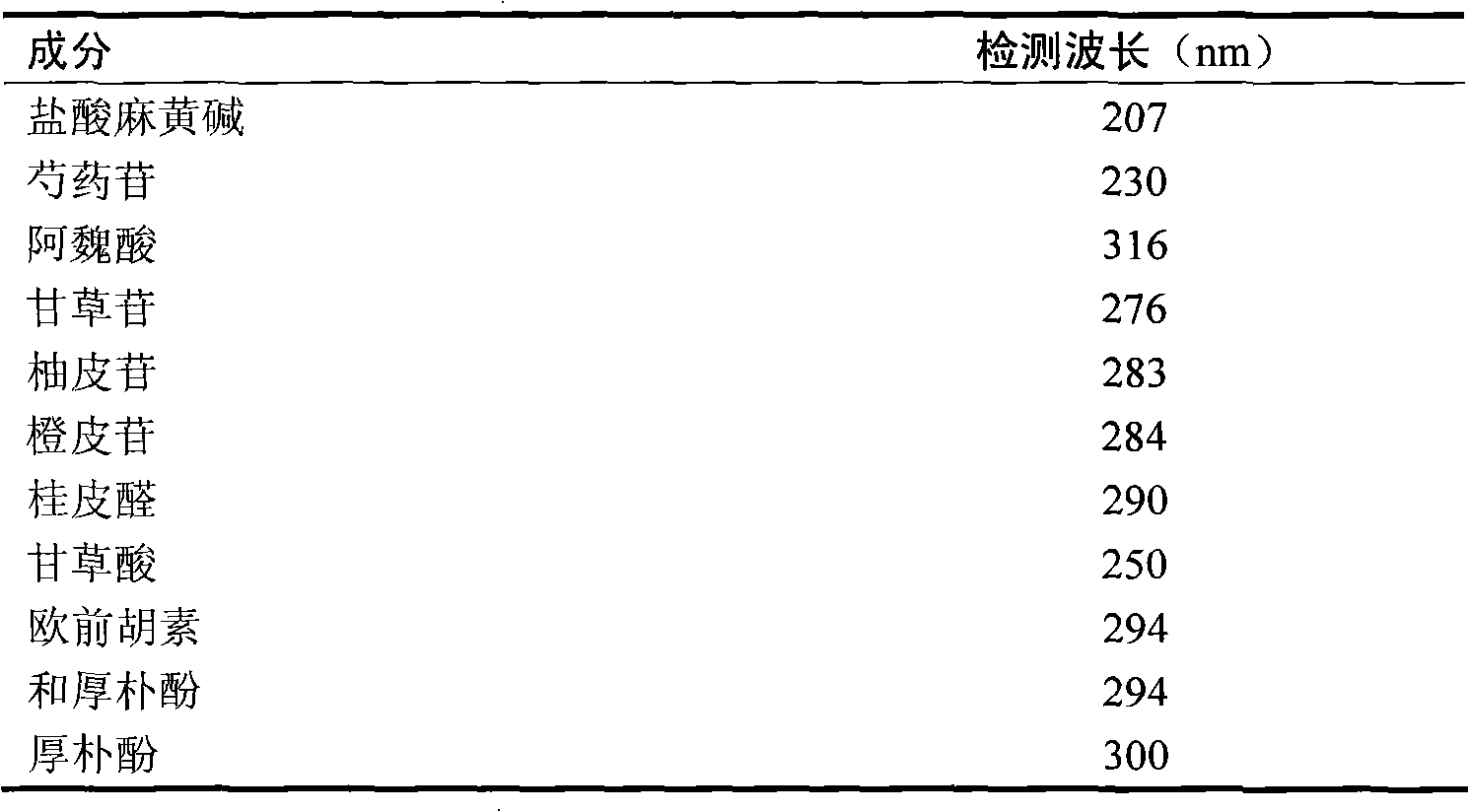
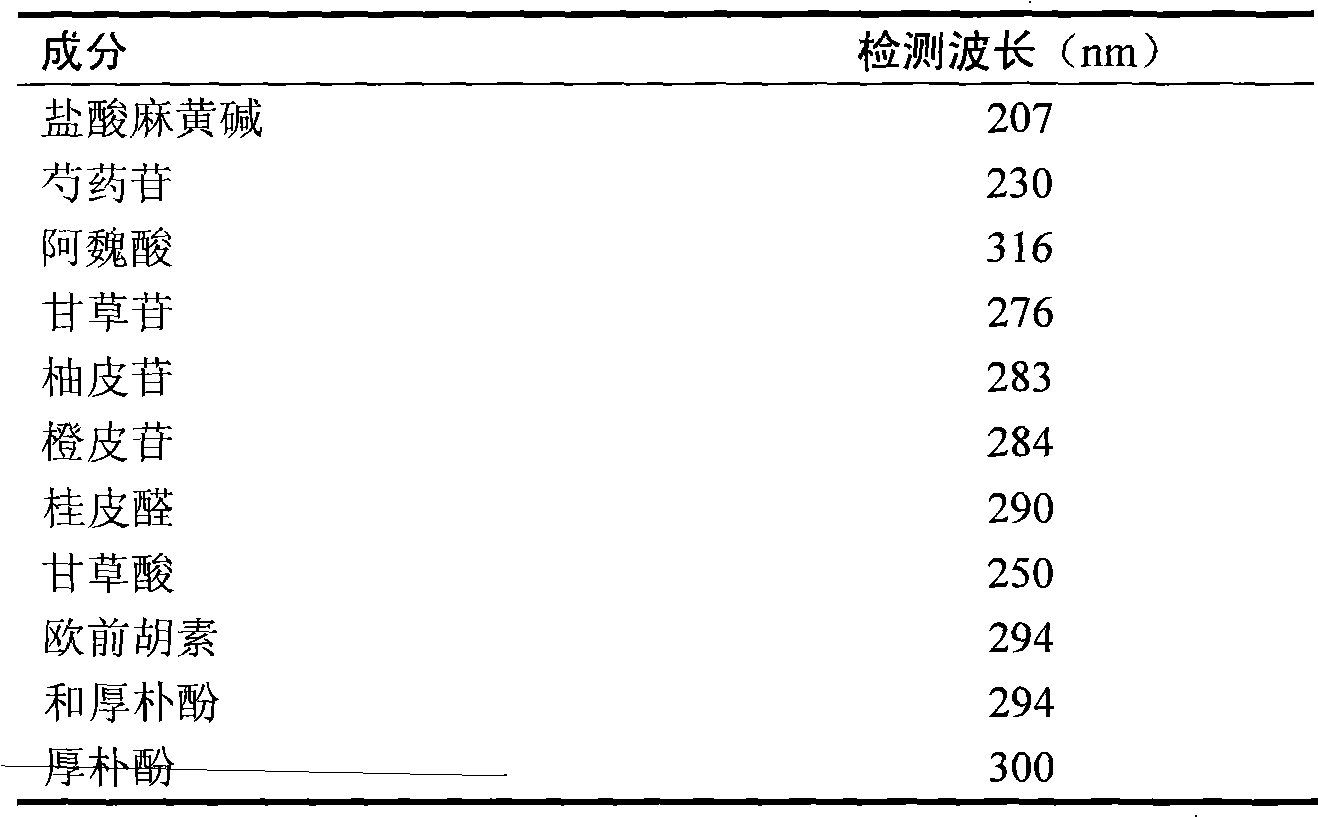
[0021] Mobile phase A is 0.35% (v / v) phosphoric acid aqueous solution; B is acetonitrile; gradient elution: 0-15min, 7-18% B; 15-38min, 18-30% B; 38-42min, 30-41% B; 42~45min, 41-55%B; 46~65min, 55-62%B; 65~75min, 62-75%B; 75~80min, 75-90%B; 80~85min, 90-93%B B; 85-95 min, 93-93% B. The column temperature is 30°C; the flow rate is 1ml / min; the detection wavelength of each control index is shown in Table 1. Flow rate: 1.0mlmin -1 .
[0022] Detection wavelength: the variable wavelength method is adopted, that is, the maximum absorption wavelength of the corresponding index component is used at the retention time position of each index component. See Table 1.
[0023] Table 1 Simultaneously detect the detection wavelength of 11 inde...
Embodiment 2
[0032] Embodiment 2 linear relationship investigation
[0033] Dilute the mixed solution of 11 kinds of reference substances to 6 concentration levels, accurately draw 5 μl into the liquid chromatograph, and record the chromatogram. Take the peak area of ephedrine hydrochloride, paeoniflorin, liquiritin, ferulic acid, naringin, hesperidin, cinnamon aldehyde, imperatorin, glycyrrhizinic acid, magnolol, and honokiol as the ordinate respectively ( Y), reference substance solution injection concentration (μl / ml) is abscissa (X) and carries out linear regression, and the regression equation is shown in Table 2. All regression equations showed a good linear relationship within the detection range (r 2 >0.9993).
[0034] Table 211 Regression data and minimum detection limit of components (n=6).
[0035]
Embodiment 3
[0036] Embodiment 3 precision investigation
[0037] Take Wujisan ultrafine powder preparation solution under the above-mentioned chromatographic conditions to carry out intra-day precision and inter-day precision tests. The intraday precision test uses the sample solutions of three concentrations to repeat the test 5 times in one day, and the interday precision test is performed once in three consecutive days. The results are shown in Table 3, and the R.S.D. of the 11 main components are all less than 5%. It shows that the method has good precision.
[0038] Table 3 precision test
[0039]
[0040]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com