Composite enzyme apophlegmatisant
A technology of compound enzyme and protease, applied in the field of medicine, can solve the problem that the marketed phlegm-relieving drugs have not yet appeared, and achieve the effect of protecting biological activity and improving ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
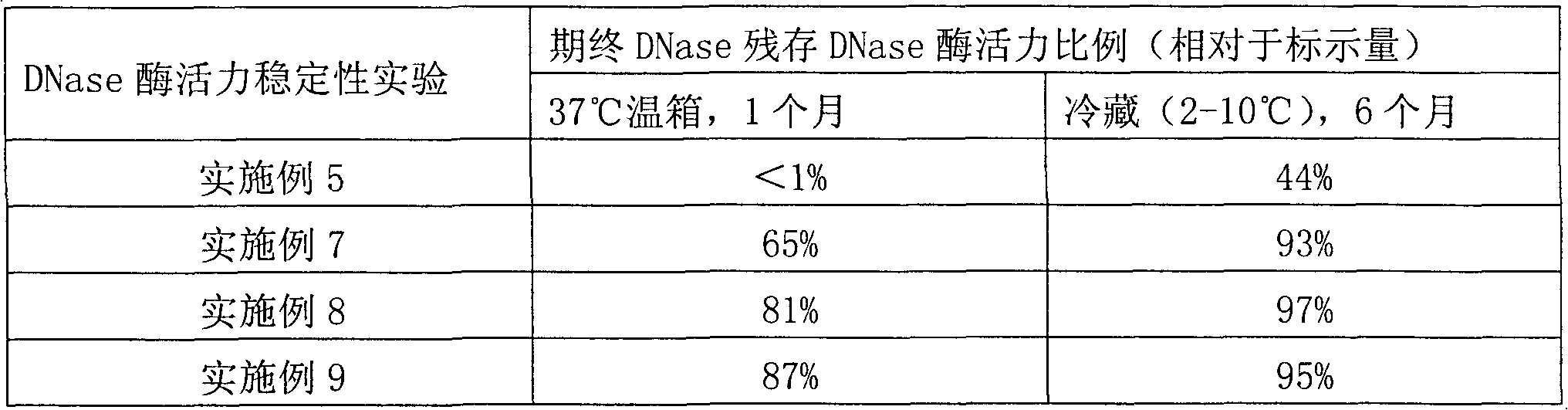
Image
Examples
Embodiment 1
[0022] Freeze-dried preparation: Dissolve 10 mg of hemolytic streptococcus dornase powder, 5 mg of bovine pancreatic deoxyribose nuclease I powder and 15 mg of subtilisin powder in 10 mL of normal saline, add 5 mg of calcium lactate and 5 g of lactose, fully dissolve and use dilute Adjust the pH value to 7.2 with sodium hydroxide, freeze-dry overnight in a -16°C freeze-dryer, and dispense. When used, dissolve the lyophilized preparation with 2-10mL of physiological saline for one dose (tracheal instillation, inhalation or oral spray).
Embodiment 2
[0024] Tablet: Add 1g porcine pancreatic DNase I powder, 1g earthworm DNase EWD1, 500mg papain powder, 100mg silkworm chrysalis protease, 200mg pepsin, 10g calcium carbonate and 35g leucine to 150g sorbitol sugar, After stirring and mixing, the tablet preparation can be combined enzyme-resolving phlegm buccal tablet (each tablet contains 10000 units of papain, 2000 units of silkworm chrysalis protease, 4000 units of pepsin, 4000 units of bovine pancreas deoxyribonuclease I and 4000 units of earthworm deoxyribonucleic acid Enzyme EWD1, take 1-2 tablets each time).
Embodiment 3
[0026] Powder spray: Dissolve 1g of citric acid in 1000mL of normal saline, add 200mg of recombinant human deoxyribonuclease I, 10mg of Serratia protease, 10mg of Streptococcus griseus protease and stir to dissolve, then add 15 grams of mannitol, 2 grams of Su Amino acid, fully dissolved and spray-dried to obtain compound enzyme powder, which is divided into hard capsules for powder inhalation. The inhalation amount of each capsule contains 2000 units of Serratia protease, 1000 units of Streptococcus griseus protease and 2000 units of Streptococcus griseus protease. Unit Recombinant Human Deoxyribonuclease I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com