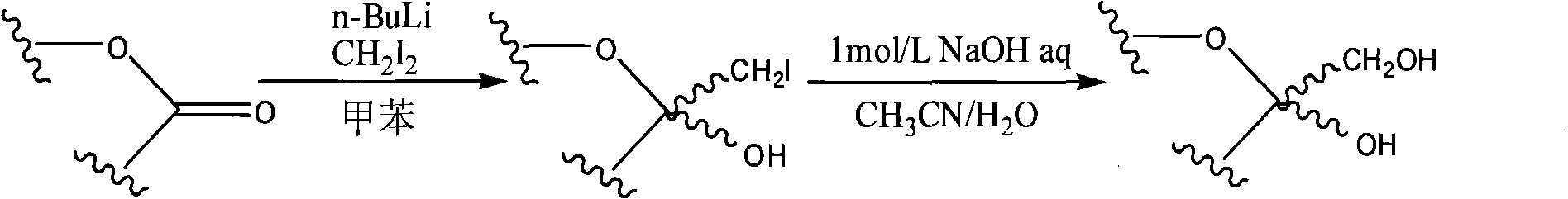Method for synthesizing rare ketohexose and ketoheptose from sugar acid lactone
A technology for rare ketohexose and sugar acid lactone, which is applied in the field of preparing rare ketohexose and heptulose, can solve the problems of difficult separation, rare raw materials, lengthy routes, etc., and achieves reduction of process steps and single protective group. , the effect of simplifying the separation operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Step 1: Preparation of 3,4,5,7-tetra-O-benzyl-1-deoxyiodomannoheptulose:
[0032] Add 1.50 g (2.79 mmol) of 2,3,4,6-tetra-O-benzyl-mannolactone, 60 mL of anhydrous toluene, and 0.53 mL of diiodomethane (6.39 mmol) into a 100 mL three-necked flask and stir well. The system was placed in an ice bath at -78°C and cooled for 10 minutes. N 2 Under protection, 3.50 mL of a n-butyllithium solution in n-hexane (5.60 mmol) with a concentration of 1.6 mol / L was slowly added dropwise thereto, and after the addition was completed, the reaction was carried out at -78° C. for 1.5 hours. Then add 15ml of saturated NH to the three-neck flask 4 Cl solution and stirred for 10 minutes to terminate the reaction. It was extracted with 2×20 ml of dichloromethane, and the organic phases were combined, dried and concentrated to obtain a yellow syrupy liquid.
[0033] Step 2: Preparation of 3,4,5,7-tetra-O-benzyl-mannoheptulose:
[0034] Dissolve the obtained yellow syrup in a 100ml one-ne...
Embodiment 2
[0041] Step 1: Preparation of 3,4,5,7-tetra-O-benzyl-1-deoxyiodomannoheptulose:
[0042] Add 1.50 g (2.79 mmol) of 2,3,4,6-tetra-O-benzyl-mannolactone, 60 mL of anhydrous toluene, and 0.53 mL of diiodomethane (6.39 mmol) into a 100 mL three-necked flask and stir well. The system was placed in an ice bath at -70°C and cooled for 20 minutes. N 2 Under protection, 1.92 mL of a n-butyllithium solution in n-hexane (3.07 mmol) with a concentration of 1.6 mol / L was slowly added dropwise thereto, and reacted at -70° C. for 0.5 hours after the addition was completed. Then add 15ml of saturated NH to the three-neck flask 4 Cl solution and stirred for 10 minutes to terminate the reaction. It was extracted with 2×20 ml of dichloromethane, and the organic phases were combined, dried and concentrated to obtain a yellow syrupy liquid.
[0043] Step 2: Preparation of 3,4,5,7-tetra-O-benzyl-mannoheptulose:
[0044] Dissolve the obtained yellow syrup in a 100ml one-necked flask with 35ml o...
Embodiment 3
[0048] Step 1: Preparation of 3,4,5,7-tetra-O-benzyl-1-deoxyiodomannoheptulose:
[0049] Add 1.50 g (2.79 mmol) of 2,3,4,6-tetra-O-benzyl-mannolactone, 60 mL of anhydrous toluene, and 0.53 mL of diiodomethane (6.39 mmol) into a 100 mL three-necked flask and stir well. The system was placed in an ice bath at -65°C and cooled for 20 minutes. N 2 Under protection, 3.99 mL of a n-butyl lithium solution in n-hexane (6.39 mmol) with a concentration of 1.6 mol / L was slowly added dropwise thereto, and after the addition was completed, the reaction was carried out at -65° C. for 1.5 hours. Then add 15ml of saturated NH to the three-neck flask 4 Cl solution and stirred for 10 minutes to terminate the reaction. It was extracted with 2×20 ml of dichloromethane, and the organic phases were combined, dried and concentrated to obtain a yellow syrupy liquid.
[0050] Step 2: Preparation of 3,4,5,7-tetra-O-benzyl-mannoheptulose:
[0051] Dissolve the obtained yellow syrup in a 100ml one-n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com