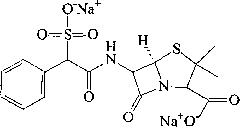
Sulbenicillin sodium liposome injection
A technology of sodium sulbenicillin lipid and sodium sulbenicillin, which is applied in the field of medicine, can solve problems such as leakage of encapsulated drugs, easy aggregation and fusion of liposomes, and achieve enhanced stability, excellent stability, and improved stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
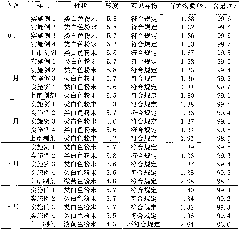
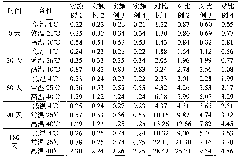
Image
Examples
Embodiment 1
[0053] The preparation of embodiment 1 sulbenicillin sodium liposome injection
[0054] Prescription (100 bottles):
[0055] Sulbenicillin Sodium 100g
[0056] Soy Lecithin 300g
[0057] Cholesterol 80g
[0058] Dipalmitoyl Phosphatidylethanolamine 20g
[0059] Vitamin E 10g
[0060] Mannitol 250g
[0061] Trehalose 250g
[0062] Preparation Process
[0063] (1) Add 300g of soybean lecithin, 80g of cholesterol, 20g of dipalmitoylphosphatidylethanolamine, and 10g of vitamin E into 1000ml of a mixed solvent of isopropanol and acetone with a volume ratio of 3:1, heat and stir to disperse evenly, and evaporate in a rotary thin film The mixed solvent was removed under reduced pressure on the apparatus to obtain a phospholipid film;
[0064] (2) Add 500ml of potassium dihydrogen phosphate-dipotassium hydrogen phosphate buffer solution with a pH value of 5.8, shake and stir to completely hydrate the phospholipid membrane at 55-60°C for 1 hour, high-speed homogeneous emulsific...
Embodiment 1
[0067] The preparation of comparative example 1 sulbenicillin sodium liposome injection
[0068] Prescription (100 bottles):
[0069] Sulbenicillin Sodium 100g
[0070] Soy Lecithin 300g
[0071] Cholesterol 80g
[0072] Vitamin E 10g
[0073] Mannitol 250g
[0074] Trehalose 250g
[0075] The preparation process was the same as in Example 1, and the sulfbenicillin sodium liposome injection not containing dipalmitoylphosphatidylethanolamine was prepared.
Embodiment 2
[0076] The preparation of embodiment 2 sulbenicillin sodium liposome injection
[0077] Prescription (100 bottles):
[0078] Sulbenicillin Sodium 200g
[0079] Soy Lecithin 1000g
[0080] Cholesterol 400g
[0081] Dipalmitoyl Phosphatidylethanolamine 200g
[0082] Vitamin E 100g
[0083] Mannitol 1000g
[0084] Trehalose 1000g
[0085] Preparation Process
[0086] (1) Add 1000g of soybean lecithin, 400g of cholesterol, 200g of dipalmitoylphosphatidylethanolamine, and 100g of vitamin E into 5000ml of a mixed solvent of isopropanol and acetone with a volume ratio of 3:1, heat and stir to disperse evenly, and evaporate in a rotary film evaporation The mixed solvent was removed under reduced pressure on the apparatus to obtain a phospholipid film;
[0087] (2) Add 3000ml of potassium dihydrogen phosphate-dipotassium hydrogen phosphate buffer solution with a pH value of 5.8, shake and stir to completely hydrate the phospholipid membrane at 55-60°C for 1 hour, high-speed hom...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com