Cyclic-ahltin type triterpenoid saponin compound and preparation method and application thereof
A technology of triterpene saponins and cycloaltin type, which is applied in the field of medicine, can solve the problems of limited application, achieve strong anti-tumor activity, simple and reliable preparation method, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
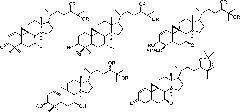
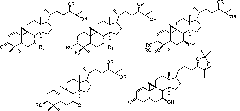
[0028] Example 1 Preparation of Cycloaltin-type triterpenoids from Pteridocarpus persicae
[0029] (1) by LC-MS technology, select the plant fern of the genus Pteridophyllaceae as the plant raw material;
[0030] (2) Utilize solvent extraction method after the plant raw material is pulverized, adopt 70% ethanol reflux to extract three times, reclaim extract under reduced pressure until alcohol concentration is lower than 2%, extract with chloroform, ethyl acetate, n-butanol extraction respectively, recover Dissolve and collect to obtain chloroform, ethyl acetate, n-butanol extract;
[0031] (3) The n-butanol extract was processed by non-polar macroporous resin (HPD-100) column chromatography, followed by water and 30%, 60%, 90% ethanol gradient elution, and each elution site was screened by LC-MS technology Determining the effective part of the 60% ethanol gradient elution part to enrich the cycloaltin-type triterpene saponins to obtain crude total saponins;
[0032] (4) The...
Embodiment 2
[0039] Embodiment 2 prepares cycloaltin-type triterpenoids from bitter horse bean
[0040] (1) Selecting the leguminous horse bean as a plant raw material by LC-MS technology;
[0041](2) After the plant raw material is pulverized, the solvent extraction method is used, and 90% ethanol is used for reflux extraction twice, and the extract is recovered under reduced pressure until the alcohol concentration is lower than 5%. After centrifugation, it is left to stand for 24 hours, and the supernatant is taken;
[0042] (3) The supernatant was processed by non-polar macroporous resin (D101) column chromatography, followed by water and 40%, 70%, 90% ethanol gradient elution, and each elution site was screened by LC-MS technology to determine 70% ethanol The gradient elution site enriches the active site of ring Altin-type triterpene saponins to obtain crude total saponins;
[0043] (4) The crude total saponins obtained in operation (3) were subjected to rapid treatment by silica ge...
Embodiment 3
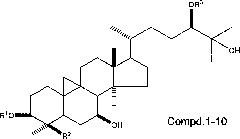
[0046] The determination of the in vitro antitumor activity of embodiment 3 ring Altin type triterpenoids
[0047] The in vitro antitumor activity of the isolated part of cycloaltin-type triterpenoids (1-10) was determined by high-throughput screening in vitro by MTT method. The experimental results are shown in Table 1.
[0048] experimental method:
[0049] 1. Cell Culture
[0050] The culture conditions of HL-60 cell line, Du145 cell line and Colon205 cell line are all RMPI 1640 culture medium, 10% fetal bovine serum, and the culture conditions of MCF-7 cell line are low-sugar DMEM medium 10% fetal bovine serum, HepG2 cells The culture condition of the strain is high-glucose DMEM culture medium with 10% fetal bovine serum, and the culture condition of A549 cell line is RMPI 1640 culture medium with 10% calf serum. All the above cell lines were cultured in 5% CO 2 , in a 37 °C incubator.
[0051] 2. Drug preparation
[0052] The determined cycloaltin-type triterpenoids...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com