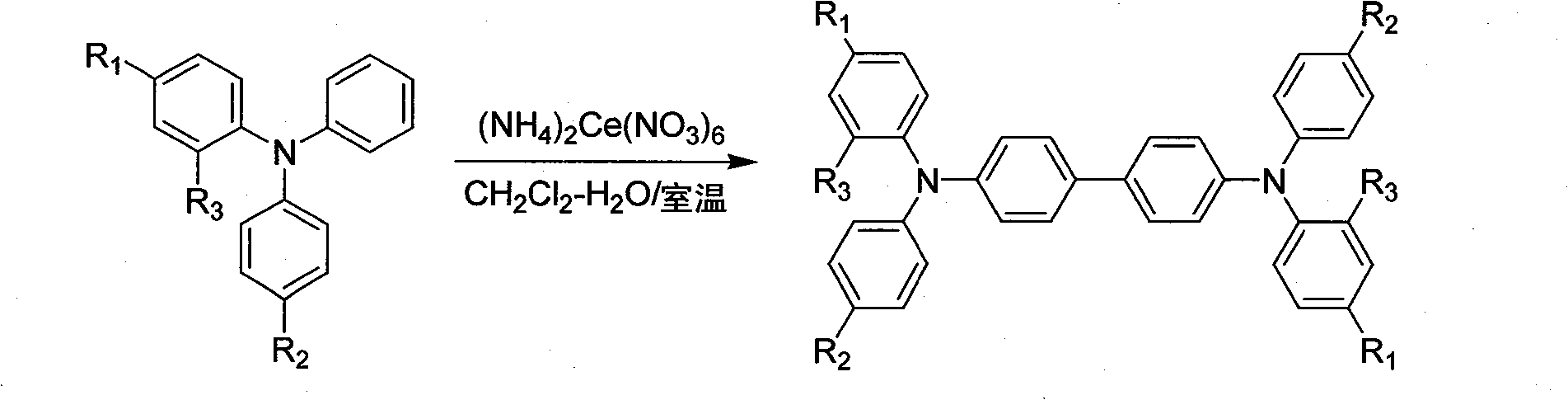
Method for synthesizing N,N,N',N'-tetra-aryl biphenyl diamine compounds through oxidative coupling of ceriums ammonium nitrate and triarylamine
A technology of aryl biphenyl diamine and ammonium cerium nitrate, which is applied in the preparation of amino compounds from amines, chemical instruments and methods, organic chemistry, etc., can solve the problems of low efficiency, harsh conditions, high cost of raw materials, etc., and achieve rapid reaction , mild conditions, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] At room temperature (25°C), in a three-liter three-necked flask equipped with mechanical stirring, dissolve 27.3 grams (0.1 moles) of 2,4-dimethyltriphenylamine in 400 milliliters of dichloromethane; stir vigorously and add After 109.6 grams (0.2 moles) of ceric ammonium nitrate, add 60 milliliters of deionized water; continue to vigorously stir for 8 minutes at room temperature; after quenching the reaction with 250 milliliters of saturated potassium carbonate solution, separate the organic phase; Extract with methane, combine the organic phases and dry with anhydrous magnesium sulfate; filter, evaporate dichloromethane in the filtrate on a rotary evaporator, pour into 200 ml of anhydrous methanol, and stir vigorously; filter and dry to obtain 25 grams of solid powder Dissolve the solid powder with 60 milliliters of toluene, filter, and after steaming off the toluene in the filtrate on a rotary evaporator, pour into about 100-150 milliliters of anhydrous methanol, stir ...
Embodiment 2
[0031] According to the method and steps described in Example 1, only 0.1 mole of 2,4-dimethyltriphenylamine was dissolved in 500 ml of dichloromethane. Finally, 21.4 grams of white powdery N, N'-diphenyl-N, N'-di(2,4-xylyl)-1,1'-biphenyl-4,4'-diamine target product was obtained (Yield 78%). Melting point 104-105°C (literature value: 108-109°C).
Embodiment 3
[0033] According to the method and steps described in Example 1, only 0.1 mole of 2,4-dimethyltriphenylamine was dissolved in 600 ml of dichloromethane. Finally, 20.6 grams of white powdery N,N'-diphenyl-N,N'-di(2,4-xylyl)-1,1'-biphenyl-4,4'-diamine target product was obtained (Yield 75%). Melting point 104-105°C (literature value: 108-109°C).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com