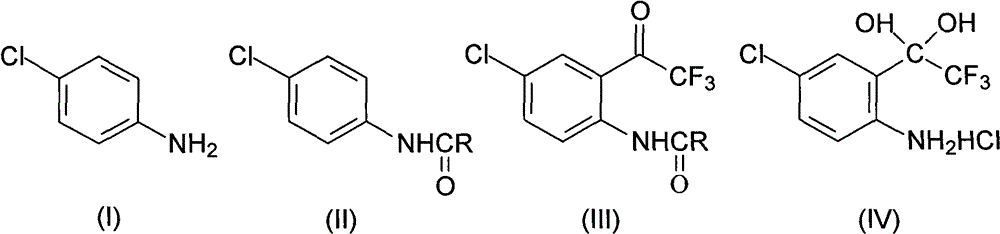
Method for synthesizing 4-chloro-2-(trifluoroacetyl)aniline hydrochloride hydrate intermediate
A technology of trifluoroacetyl and aniline hydrochloride, which is applied in the preparation of organic compounds, chemical instruments and methods, and the production of bulk chemicals, and can solve problems such as troublesome preparation of butyllithium, incomplete recovery, and increased use costs , to achieve the effect of low price, low cost, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
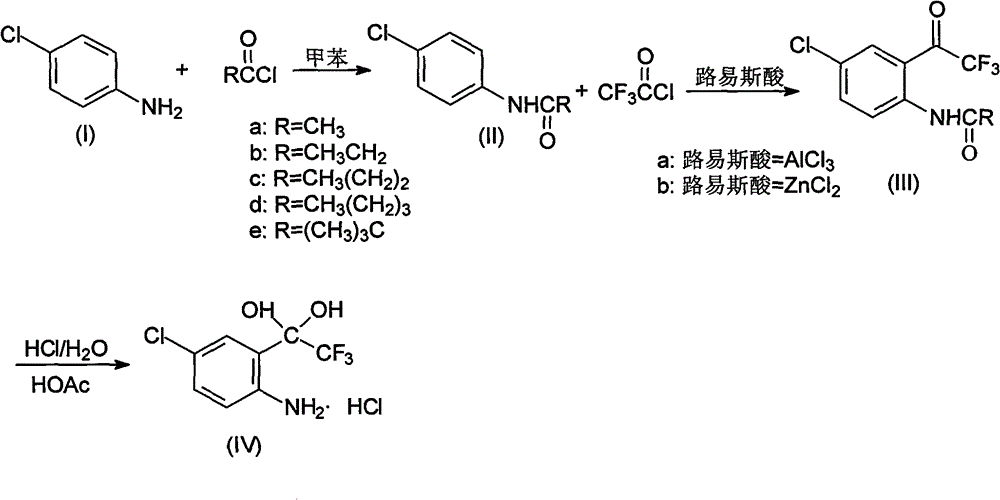
[0026] Put 80.3g (0.63mol) of p-chloroaniline and 500ml of toluene in a 1000ml flask equipped with a stirrer, dropping funnel and thermometer, cool down to 0-5°C, and put 90.8g (0.68mol) of 30% sodium hydroxide Solution, controlled at 5-15°C, add 51.1g (0.65mol) acetyl chloride dropwise, after 0.5h drop, keep warm at 5-15°C for 1h, separate layers, wash the organic layer twice, cool to -5°C and keep warm for 2h , filtered with suction, washed the filter cake with water, drained, vacuumed at -0.085Mpa, and dried below 70°C for 24 hours to obtain 103.2g of compound 4-chloro-N-acetylaniline, with a content of 99.0%, a moisture content of ≤0.3%, and a yield of 96.6%.
[0027] In this embodiment, propionyl chloride, butyryl chloride, valeryl chloride or pivaloyl chloride are used instead of acetyl chloride to achieve the same effect.
Embodiment 2
[0029] Put 80.3g (0.63mol) of p-chloroaniline and 500ml of toluene in a 1000ml flask equipped with a stirrer, dropping funnel and thermometer, cool down to 0-5°C, and put 90.8g (0.68mol) of 30% sodium hydroxide Solution, controlled at 5-15°C, add 83.2g (0.69mol) of pivaloyl chloride dropwise, after 1h dripping, keep at 5-15°C for 1h, separate layers, wash the organic layer twice, cool to -5°C and keep for 2h , filtered with suction, washed the filter cake with water, drained, vacuumed at -0.085Mpa, and dried below 70°C for 24 hours to obtain 103.8g of compound 4-chloro-N-acetylaniline, with a content of 99.3%, a moisture content of ≤0.2%, and a yield of 97.2%.
[0030] 2) Synthesis of 4-chloro-2-(trifluoroacetyl)aniline hydrochloride hydrate (Ⅳ)
Embodiment 3
[0032] Add 133.5g (1.0mol) of anhydrous aluminum trichloride and 200g of n-hexane into the reaction flask, stir and mix, cool down to -20°C, slowly introduce 132.5g (1.0mol) of trifluoroacetyl chloride, and mix 169.5g ( 1.0mol) of 4-chloro-2-acetylaniline was dissolved in 600g of n-hexane, and then slowly dripped into the above system at -20°C for 5.5 hours. After the addition was completed, it was naturally raised to room temperature and kept stirring for 2 hours. . After the reaction was complete, the reaction mixture was dropped into 400 g of ice water, and the temperature was controlled below 15° C., stirred for 15 min after the drop was completed, and allowed to stand to separate into layers. The organic layer was washed with water until neutral, and the aqueous layers were combined and extracted twice with 200 g of n-hexane. The organic layers were combined and concentrated to dryness under reduced pressure to obtain 270.6 g of oily N-acetyl-4-chloro-2-(trifluoroacetyl)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com