Preparation method of beta-aluminum fluoride with high specific surface area
A high specific surface area, aluminum fluoride technology, applied in aluminum fluoride, aluminum halide and other directions, can solve the problems of inconvenient operation and control, complex preparation process, high corrosion of the device, etc., to achieve easy operation and control, high purity, The effect of less corrosiveness of the device
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 100g specific surface area is 350m 2 g -1 γ-alumina as raw material, sucrose as carbon source, 80ml of sucrose solution with a mass fraction of 25% was added to γ-alumina, soaked for 3 hours, and dried at 400°C under N 2 Calcining under the atmosphere for 3 hours, impregnating, drying and calcining the carbon-containing γ-alumina after the first carbon filling, obtained the carbon-containing γ-alumina after the second carbon filling. Put the charcoal-containing γ-alumina in a fixed bed reactor and feed N 2 The mixed gas of HF gas and HF gas is used for fluorination treatment, in which HF gas and N 2 The molar ratio is 4, the fluorination temperature is 300°C, and the fluorination time is 6h. The carbon-containing aluminum fluoride after the fluorination treatment is impregnated with 5% potassium nitrate by equal volume, dried, put into a high-temperature furnace, and calcined at 425° C. for 6 hours in an oxygen atmosphere. The obtained substance was heat-treated wit...
Embodiment 2
[0028] The preparation method of the sample of the present invention is basically the same as Example 1, the difference is that the specific surface area is 302m 2 g -1 γ-alumina as raw material.
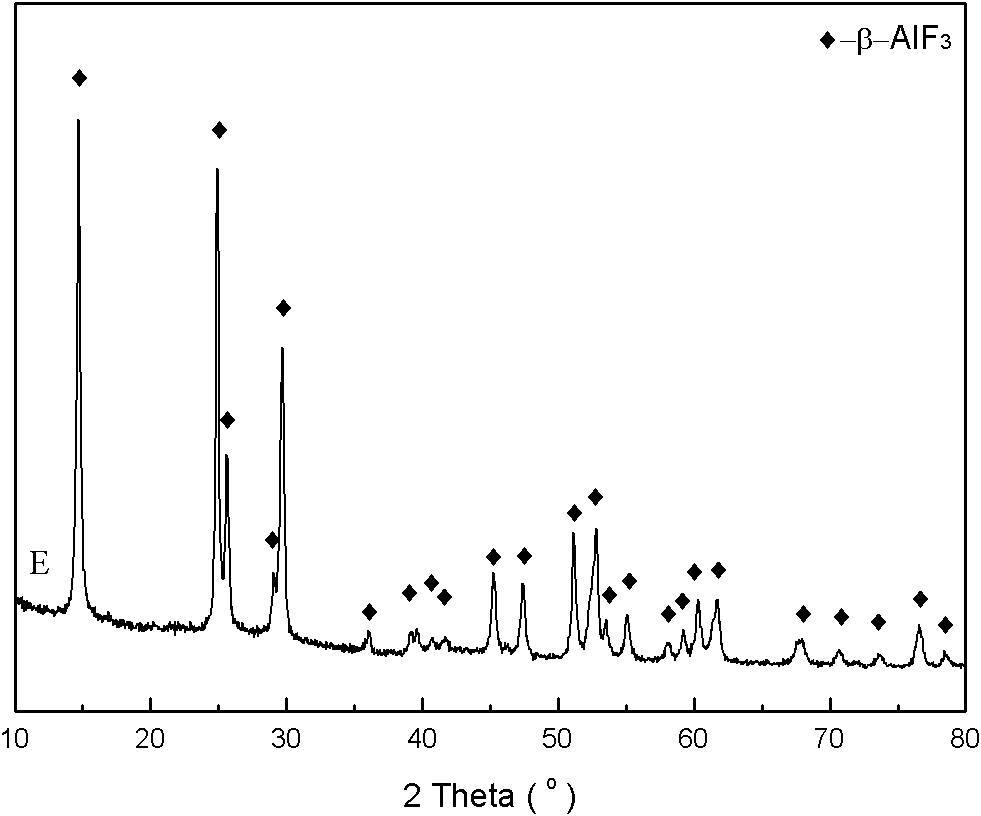
[0029] The product made in this embodiment is found to be β-aluminum fluoride substance by X-ray diffraction pattern, and the specific surface area measured by BET low-temperature nitrogen adsorption is 94m 2 g -1 .
Embodiment 3
[0031] The preparation method of the sample of the present invention is basically the same as Example 1, the difference is that the specific surface area is 251m 2 g -1 γ-alumina as raw material.
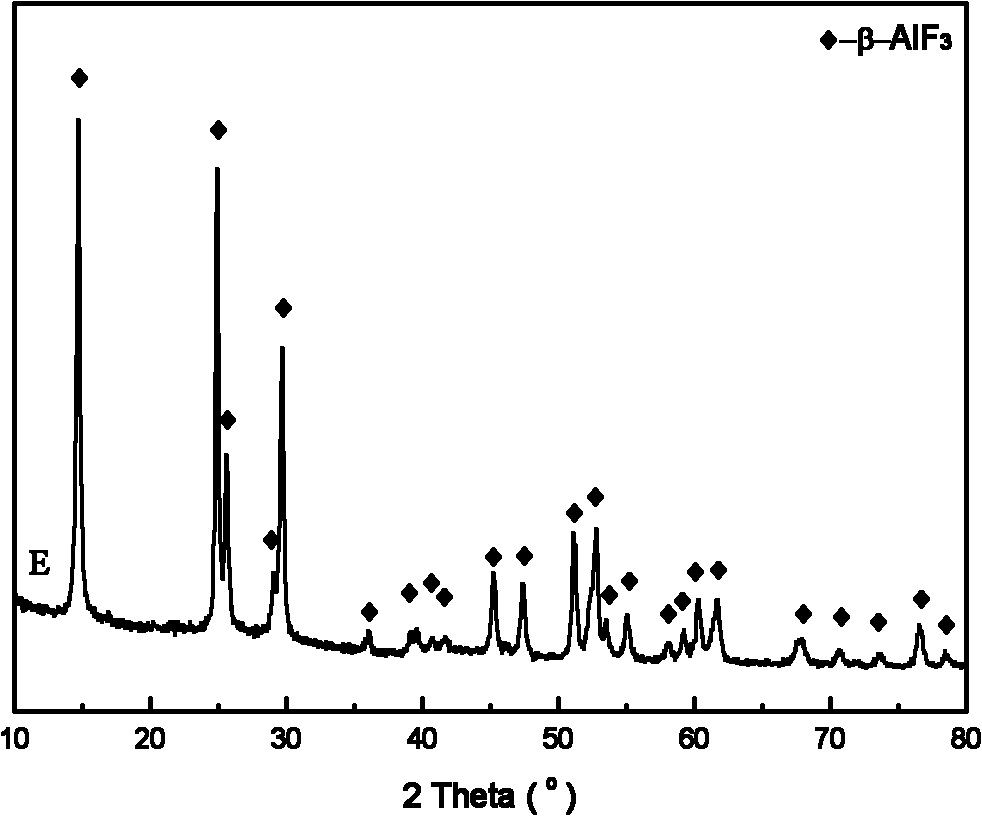
[0032] The product that this embodiment makes is found to be β-aluminum fluoride substance by X-ray diffractogram, and the specific surface area measured by BET low-temperature nitrogen adsorption is 84m 2 g -1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com