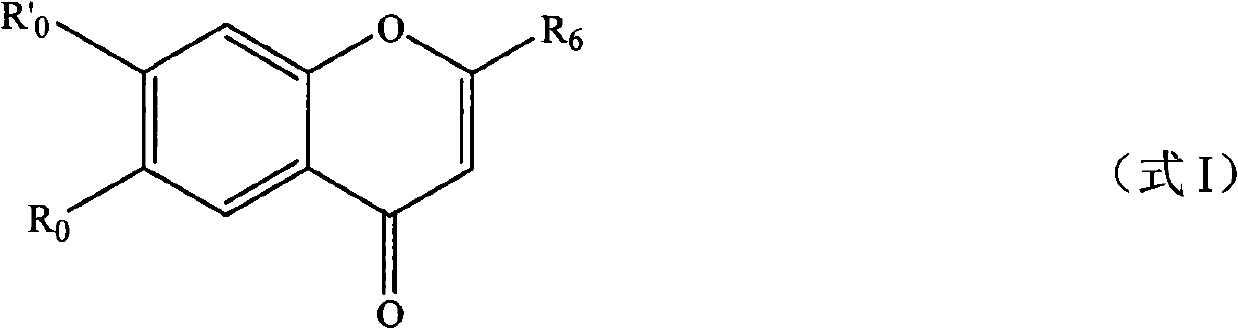
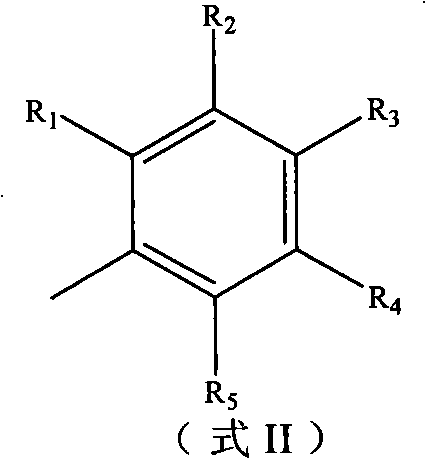
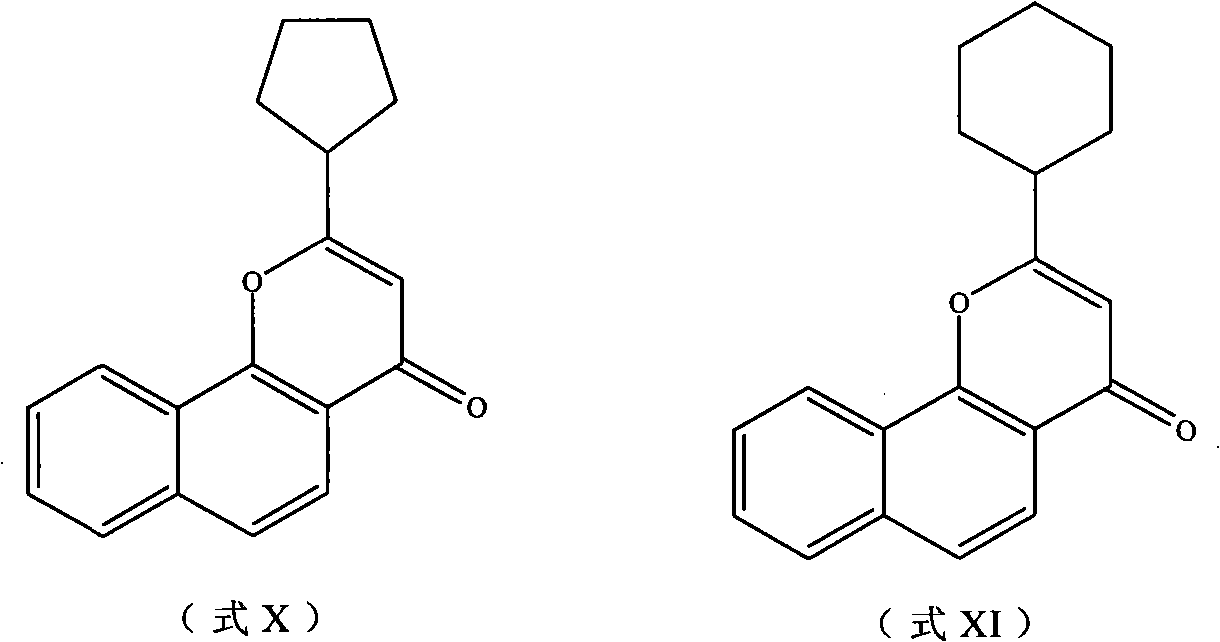
Derivatives of flavonoids compounds and preparation method and application thereof
A technology of flavonoids and derivatives, applied in medical preparations containing active ingredients, organic chemistry, drug combinations, etc., can solve the problems of urgent research, strong side effects and drug resistance, and achieve good tumor activity and tumor inhibition activity , the effect of easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1. Preparation of 3', 5'-dimethoxy-6-chloroflavone
[0033] Step 1): Add 50ml of ice water, 10mmol of p-chlorophenol, and 15mmol of sodium hydroxide into a 100mL flask, slowly add 15mmol of acetic anhydride under stirring conditions, continue to stir at room temperature for 2.5h, stop the reaction, use ethyl acetate to extract three times, anhydrous Dried over magnesium sulfate and rotary steamed under pressure to obtain the desired compound -4-chlorophenol acetate.
[0034] Step 2): Add 5mmol acetic acid-4-chlorophenol ester, 7.5mmol anhydrous aluminum chloride, 7.5mmol sodium chloride into a 100ml beaker and react for 2h under melting and stirring, then add ice water, extract, dry, and rotary steam under reduced pressure to obtain Rearranged 2-acetyl-4-chlorophenol.
[0035] Step 3): Add 5ml of anhydrous pyridine and 1.5mmol of 3,5-dimethoxybenzoyl chloride into a 100ml flask, add 1mmol of 2-acetyl-4 chlorophenol under stirring conditions, continue to reac...
Embodiment 2
[0038] Embodiment 2, preparation compound 2'-chloro-4'-nitro-6-isopropylflavone
[0039] Step 1): Add 50ml of ice water, 10mmol of p-isopropylphenol, and 15mmol of sodium hydroxide into a 100mL flask, slowly add 15mmol of acetic anhydride under stirring conditions, continue to stir at room temperature for 2.5h, stop the reaction, and extract it roughly three times with ethyl acetate, Dry over anhydrous magnesium sulfate, and pressurize and rotary evaporate to obtain the desired compound -4-isopropylphenol acetate.
[0040] Step 2): Add 5mmol acetic acid-4-isopropylphenol ester, 7.5mmol anhydrous aluminum chloride, 7.5mmol sodium chloride into a 100ml beaker and react for 2h under melting and stirring, then add ice water, extract, dry, spin under reduced pressure Evaporation affords the rearranged 2-acetyl-4-isopropylphenol.
[0041] Step 3): Add 5ml of anhydrous pyridine and 1.5mmol of 2-chloro-4-nitrobenzoyl chloride into a 100ml flask, add 1mmol of 2-acetyl-4 isopropylpheno...
Embodiment 3
[0044] Embodiment 3, preparation 2'-chloro-4'-amino-6-isopropylflavone
[0045] Add 0.5mmol of 2'-chloro-4'-nitro-6-isopropylflavone prepared in Example 2 to a 50ml round low flask, then add 15ml of glacial acetic acid, stir under ice bath, add 2mmol of zinc powder, 0.5h After the reaction was complete, the unreacted zinc powder was filtered out, and the glacial acetic acid was removed under reduced pressure. The residue is separated by column chromatography, and the silica gel column used is purchased from Beijing Xinweier Glass Instrument Co., Ltd. The chromatographic column model is C384630C, and the length and internal diameter ratio is 305mm: 40mm. When used to separate the product of the present invention, the eluent used is Ethyl acetate (V): Petroleum ether (V)) = 1:5 ~ 1:10 mixed solution, the eluent flows under normal pressure (2ml / min), detects the effluent according to TLC thin layer chromatography, and depressurizes The product obtained by rotary evaporation is w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com