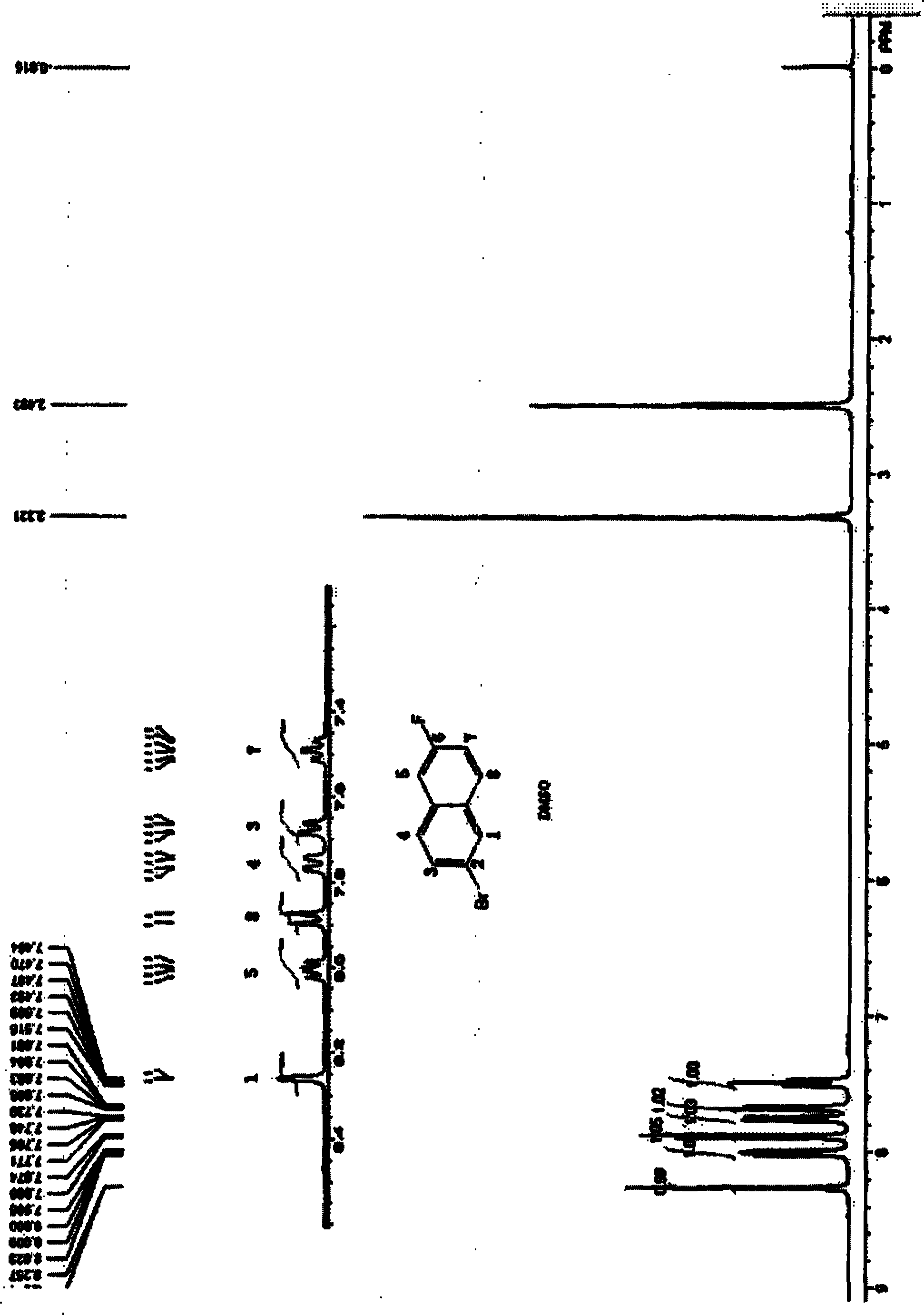
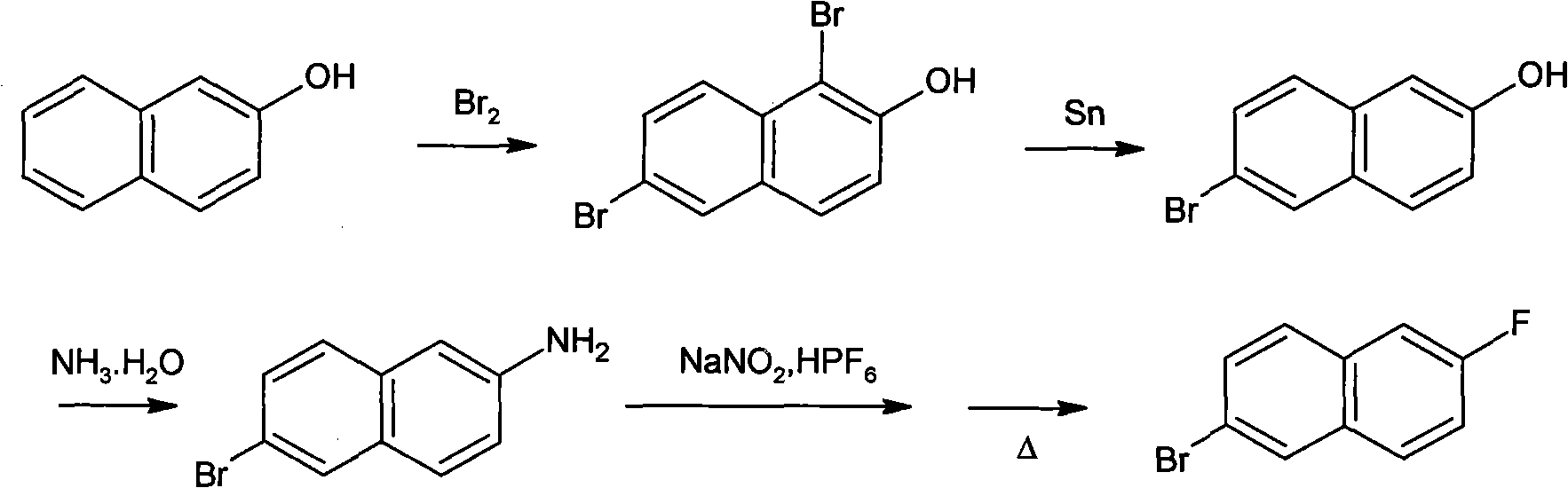
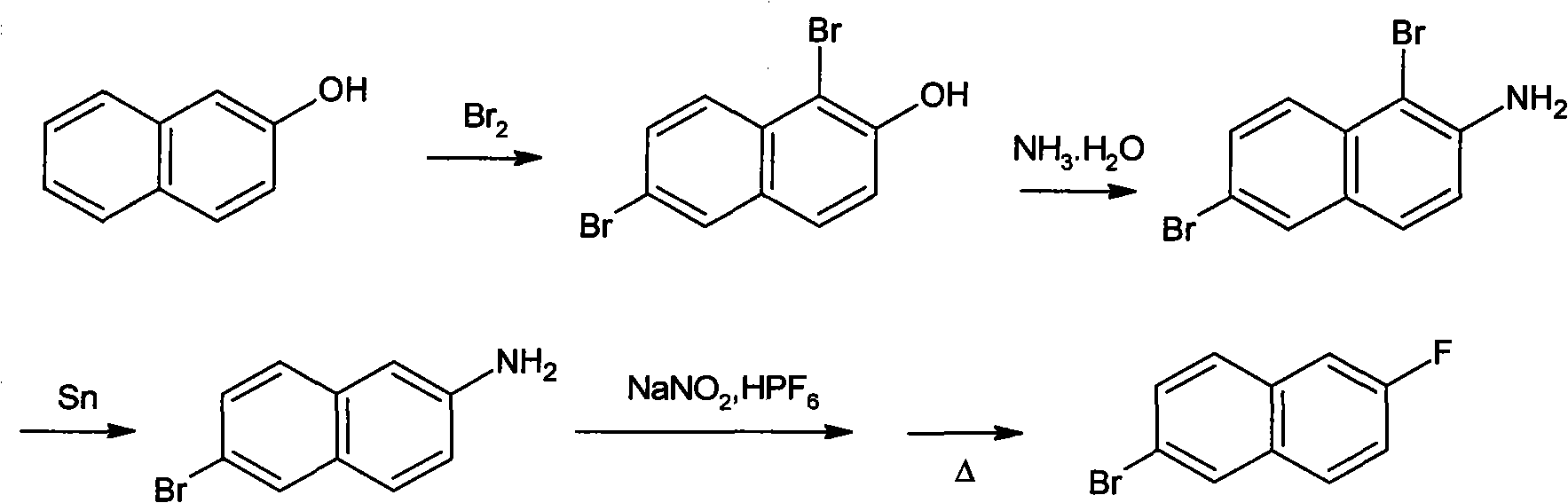
Preparation method of 2-bromo-6-fluoronaphthalene
A technology of fluoronaphthalene and naphthylamine, which is applied in the field of pharmaceuticals, can solve problems such as equipment corrosion, seriousness, and harsh equipment requirements, and achieve the effect of simple routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Synthesis of 6-bromo-2-naphthylamine:
[0034] In a 2000ml three-necked flask equipped with a dropping funnel, a thermometer, a stirring device and a reflux condenser, add 1100ml of glacial acetic acid and 57.47g (0.25mol) of Turmeric's acid, start stirring, and heat to 70°C to dissolve the Turmeric's acid . 80 g (0.5 mol) of liquid bromine was added dropwise through the dropping funnel, and the temperature of the reactant was kept between 70-72° C. during the dropwise addition, and the dropwise addition was completed in about 1 hour. After the dropwise addition, the temperature was raised to reflux, and stirring was continued for 1.5 hours under reflux to complete the reaction. After the reaction finishes, the temperature of the reaction mixture is reduced to 65°C, adding 29.8g (0.251mol) metal tin powder and 340ml mass concentration is 35% hydrochloric acid, then the temperature of the reaction mixture is raised to reflux, and continues to stir for 2 Hour. Afte...
Embodiment 2
[0040] Carry out by the same method of embodiment 1, difference is that the thermal decomposition of the diazonium salt in the step (3) is carried out in the silicone oil that boiling point is in the range of 250-300 ℃, the product yield that obtains is 56.9%, HPLC The detection purity is 99.5%.
Embodiment 3
[0042] Carry out by the same method of embodiment 1, difference is, the thermal decomposition of the diazonium salt in the step (3) is carried out in liquid paraffin (C16-C20 normal alkanes), and the product yield that obtains is 58.4%, and HPLC detects 99.7% purity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com