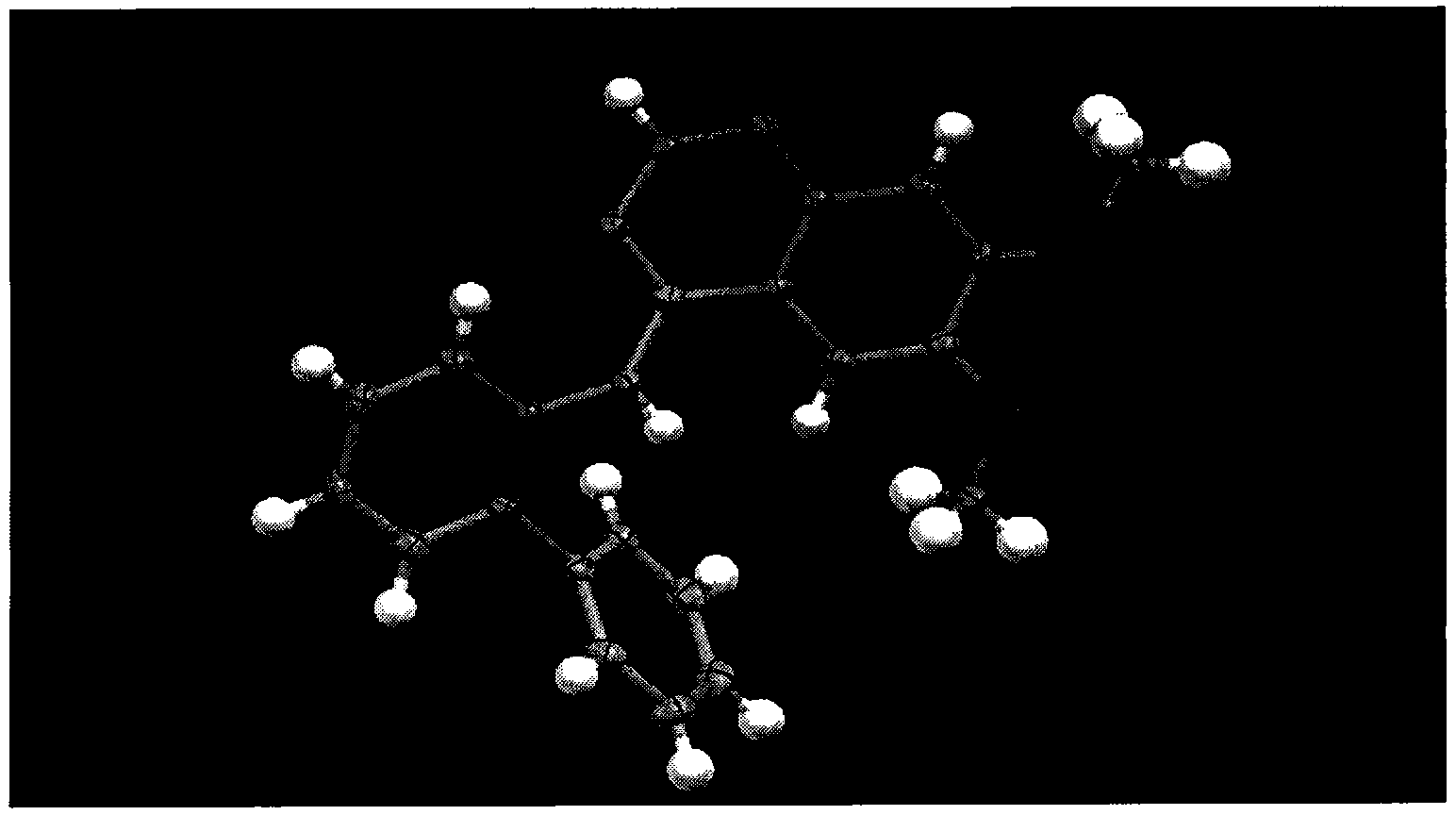
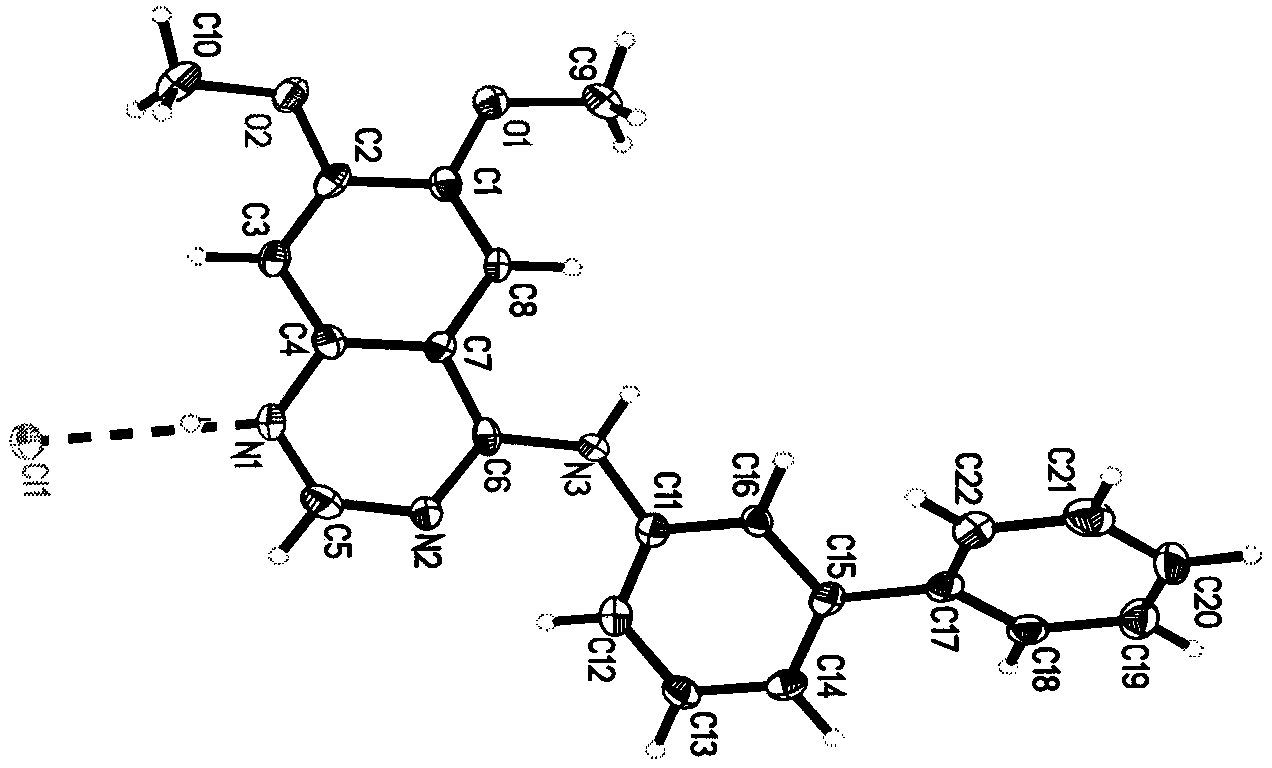
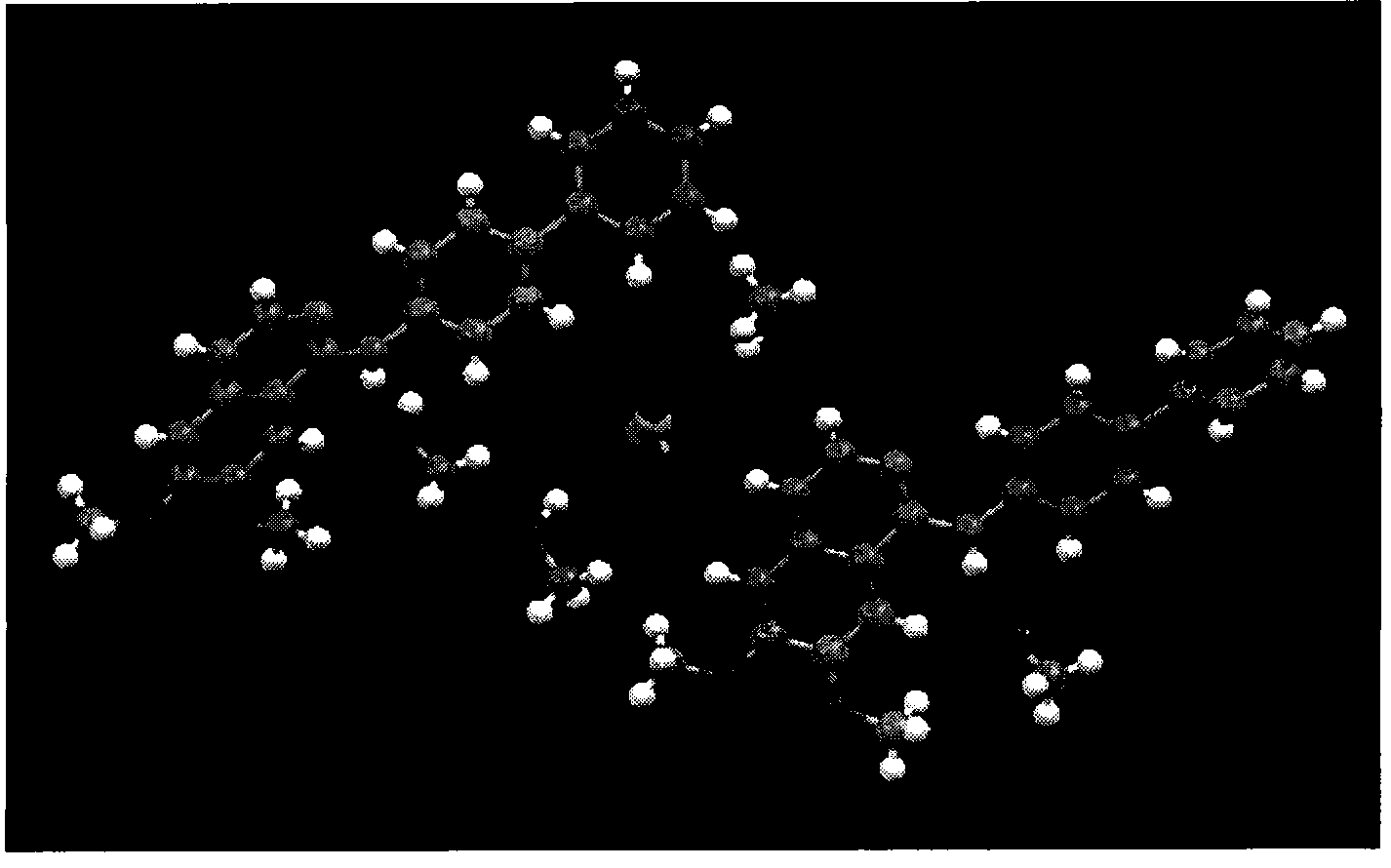
Tyrosine protein kinase inhibitor and preparation method thereof
A kinase inhibitor, tyrosine protein technology, applied in pharmaceutical formulations, medical preparations containing active ingredients, organic chemistry, etc., can solve the problems of poor water solubility, large toxic and side effects of cytotoxic drugs, etc. Good inhibitory activity and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] For example, the preparation method of the compound represented by general formula (26)-(27) may include:
[0049] (1) One of nitro-substituted 1,2-diaminobenzene and a saturated fatty acid with 1-3 carbon atoms, 2-pyridyl formic acid and 2-pyridyl acetic acid in hydrochloric acid aqueous solution, phosphoric acid and polymer In one or several solutions of phosphoric acid, under reflux conditions, reflux for 3-5 hours, cool, and adjust the pH of the reaction mixture to 9-10, filter, wash and recrystallize to obtain nitro-substituted benzene and imidazole; the 1,2-diaminobenzene substituted with nitro and the molar ratio of one of saturated fatty acid, 2-pyridyl formic acid and 2-pyridyl acetic acid with carbon number of 1-3 can be 1:1 -1:3, based on the amount of 1 gram of nitro-substituted 1,2-diaminobenzene, the volume of the acid or acid aqueous solution is 8-20 milliliters, and the concentration of the aqueous hydrochloric acid solution is generally 5 mol / liter of h...
Embodiment 1
[0066] This example is used to illustrate the preparation of the tyrosine protein kinase inhibitor provided by the present invention.
[0067]
[0068] The first reactant (1) (purchased from Shanghai Fude Chemical Co., Ltd.) and the second reactant (2) (purchased from Sigma Company) in the above reaction formula are heated to reflux in isobutanol, and the first reaction The product is 4-chloro-6,7-dimethoxyquinazoline, and the second reactant is respectively The molar ratio of the first reactant and the second reactant is 1:1, based on the total amount of the first reactant and the second reactant being 100 mg, the consumption of isobutanol is 15 milliliters; The conditions of heating to reflux include a reflux temperature of 80°C and a reflux time of 4 hours to obtain compounds LQ1001, LQ1002, LQ1003, LQ1004, LQ1005, LQ1006, LQ1007, LQ1008, LQ1009, and LQ10010.
[0069] The compounds prepared above were characterized by nuclear magnetic resonance and mass spectrometry ...
Embodiment 2
[0092] This example is used to illustrate the preparation of the tyrosine protein kinase inhibitor provided by the present invention.
[0093]
[0094] Be the first reactant (1) 4-chloro-6,7-dimethoxyquinazoline and the second reactant (3) 5-aminophenanthroline ( purchased by Sigma Company), in the catalyst Pd 2 (dba) 3 、t(Bu) 3 In the presence of P and t-BuONa (the catalyst Pd 2 (dba) 3 、t(Bu) 3 The molar ratio of P and t-BuONa to the first reactant (1) is 0.0125:0.075:3:1), heated to reflux in toluene for 28 hours, cooled to room temperature, filtered, washed with toluene and ether until the unreacted The raw material was removed and recrystallized in ethanol to obtain a pale yellow solid. The target product LS1001 was characterized by NMR and mass spectrometry.
[0095] LS1001: C 22 h 17 N 5 o 2 Calc.MW=383.3, ESI-MS (m / z): [M+H] + 384.1.
[0096] 1 H NMR: (deuterated DMSO) δ (ppm): 10.1197 (s, 1H), 9.1047 (d, 1H), 9.0564 (s, 1H), 8.4577 (m, 2H), 8.1917 (s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com