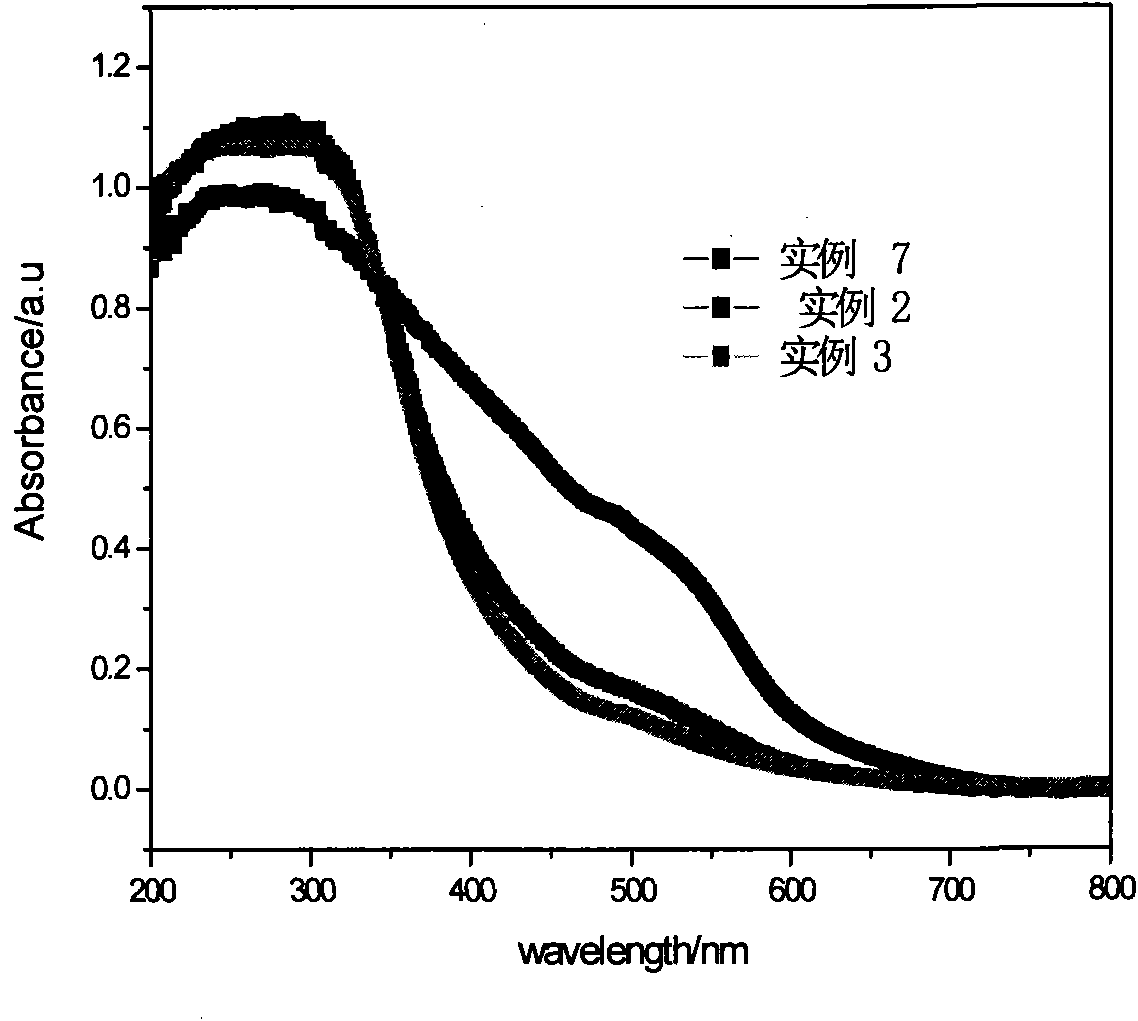
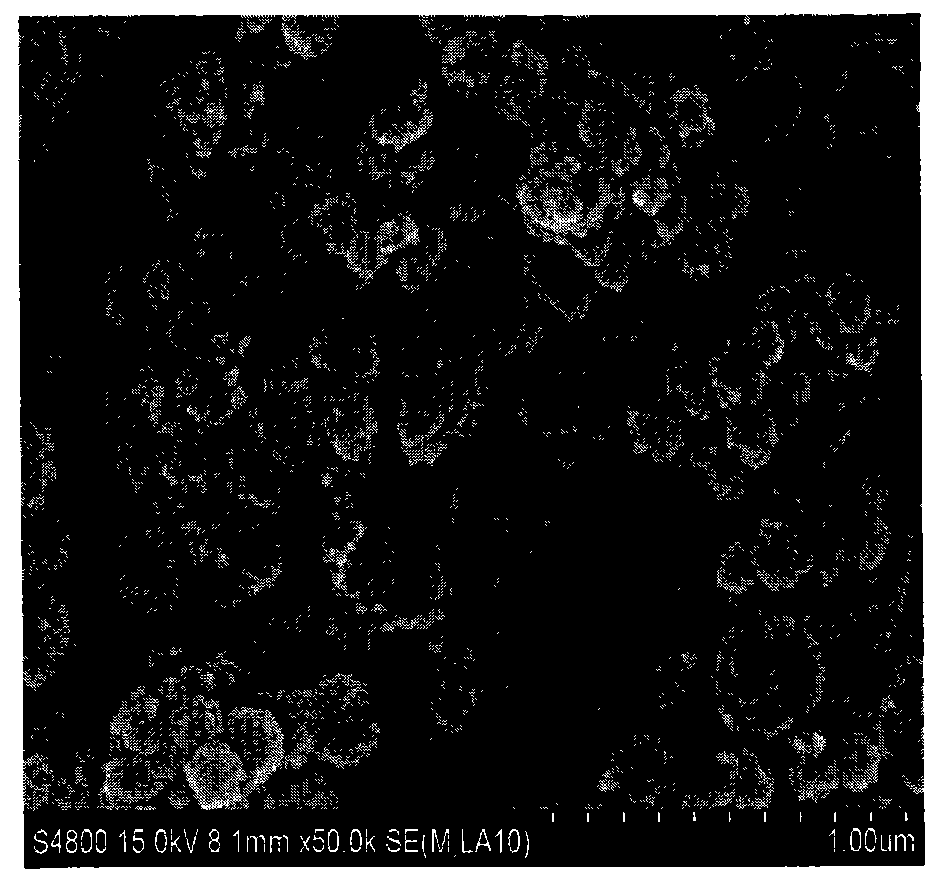
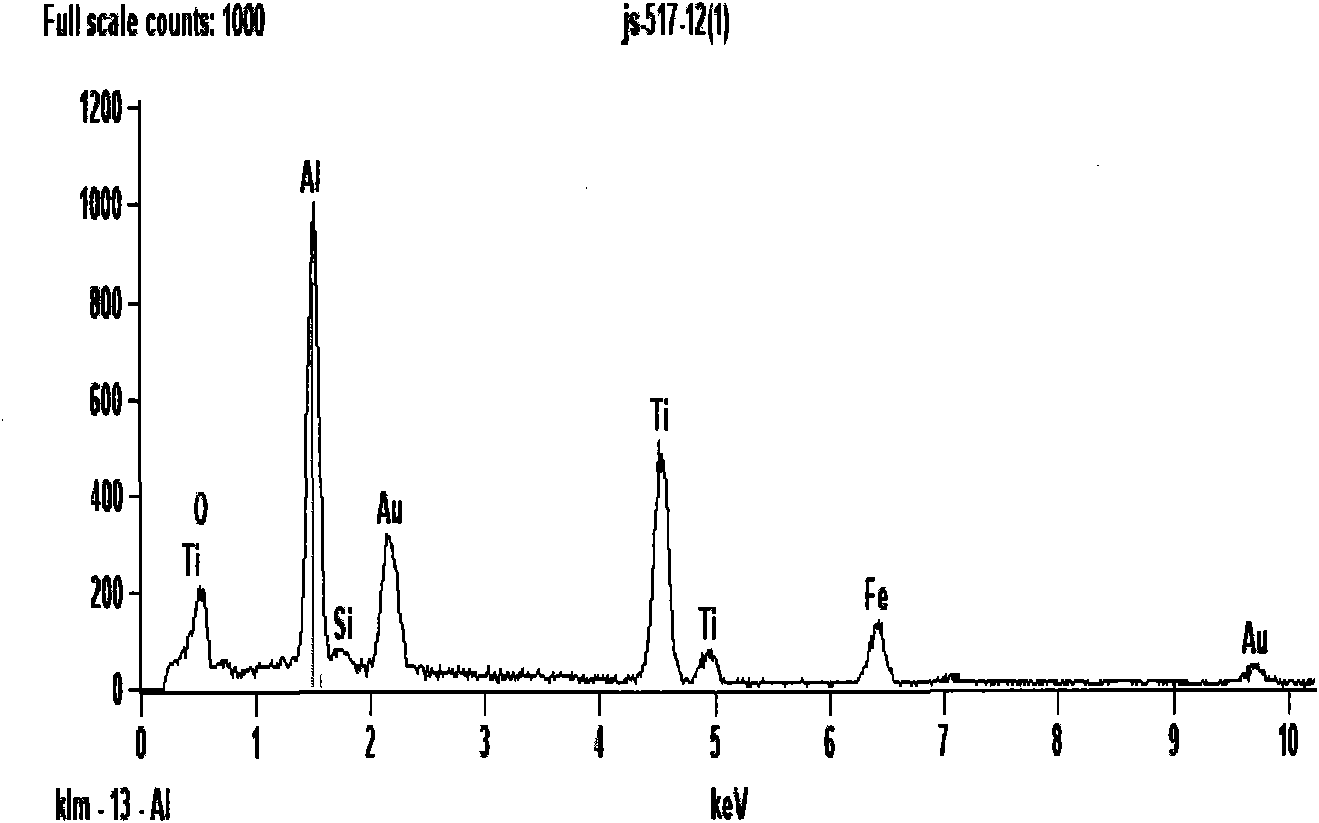
Method for preparing Fe3+doped TiO2 hollow sphere catalyst and application thereof
A technology of hollow spheres and catalysts, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxides/metal hydroxide catalysts, etc., can solve the problems of no literature reports, etc., to improve utilization rate and reduce The effect of degradation cost and degradation efficiency improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0022] 1) Weigh 0.00119g of ferric chloride hexahydrate into a three-necked flask with stirring, measure 30ml of absolute ethanol, and prepare an ethanol solution of ferric chloride. Then weigh 0.06g of nano-carbon spheres, the diameter of which is in the range of 100-200nm, add 0.3ml of distilled water into it, and ultrasonically disperse until evenly mixed.
[0023] 2) Add 30 ml of absolute ethanol into a dry constant pressure dropping funnel, measure 0.3 ml of n-butyl titanate and add it to prepare an ethanol solution of n-butyl titanate.
[0024] 3) Slowly add n-butyl titanate ethanol solution into the mixed solution obtained in step 1) under stirring condition, stir, and heat to reflux at 80° C. for 6 h. After the reflux is completed, continue to stir for 30 minutes, centrifuge, wash, and dry to obtain Fe 3+ Carbon / titania core-shell particles.
[0025] 4) with the Fe obtained in step 3) 3+ Doped carbon / titanium dioxide core-shell particles are burned in a mufur furnac...
example 2
[0028] 1) Weigh 0.00238g of ferric chloride hexahydrate into a three-necked flask with stirring, measure 50ml of absolute ethanol, and prepare an ethanol solution of ferric chloride. Then weigh 0.06g of nano-carbon spheres, the carbon spheres have a diameter ranging from 100 to 200nm, add 0.5ml of distilled water into it, and ultrasonically disperse until they are evenly mixed.
[0029] 2) Add 50 ml of absolute ethanol into a dry constant-pressure dropping funnel, measure 0.3 ml of n-butyl titanate and add it to prepare an ethanol solution of n-butyl titanate.
[0030] 3) Slowly add n-butyl titanate ethanol solution into the mixed solution obtained in step 1) under stirring condition, stir, and heat to reflux at 80° C. for 6 h. After the reflux is completed, continue to stir for 30 minutes, centrifuge, wash, and dry to obtain Fe 3+ Doped carbon / titania core-shell particles.
[0031] 4) with the Fe obtained in step 3) 3+ Fe 3+ Doped TiO 2 Open or closed hollow sphere photo...
example 3
[0034] 1) Weigh 0.0119g of ferric chloride hexahydrate into a three-necked flask with stirring, measure 80ml of absolute ethanol, and prepare an ethanol solution of ferric chloride. Then weigh 0.6g of nano-carbon spheres, the diameter of which is in the range of 100-200nm, add 3ml of distilled water into it, and ultrasonically disperse until uniformly mixed.
[0035] 2) Add 80 ml of absolute ethanol to a dry constant pressure dropping funnel, measure 3 ml of n-butyl titanate and add it to prepare an ethanol solution of n-butyl titanate.
[0036] 3) Slowly add n-butyl titanate ethanol solution into the mixed solution obtained in step 1) under stirring condition, stir, and heat to reflux at 90° C. for 5 h. After the reflux is completed, continue to stir for 2 hours, centrifuge, wash, and dry to obtain Fe 3+ Doped carbon / titania core-shell particles.
[0037] 4) with the Fe obtained in step 3) 3+ Fe 3+ Doped TiO 2 Open or closed hollow sphere photocatalyst, wherein Fe / Ti mol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com