Production method of macrocrystalline hexagonal boron nitride
A technology of hexagonal boron nitride and production method, applied in chemical instruments and methods, nitrogen compounds, inorganic chemistry, etc., can solve the problems of decreased product yield, high cost, decreased product purity, etc., to ensure mixing uniformity, production Low cost and the effect of reducing side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
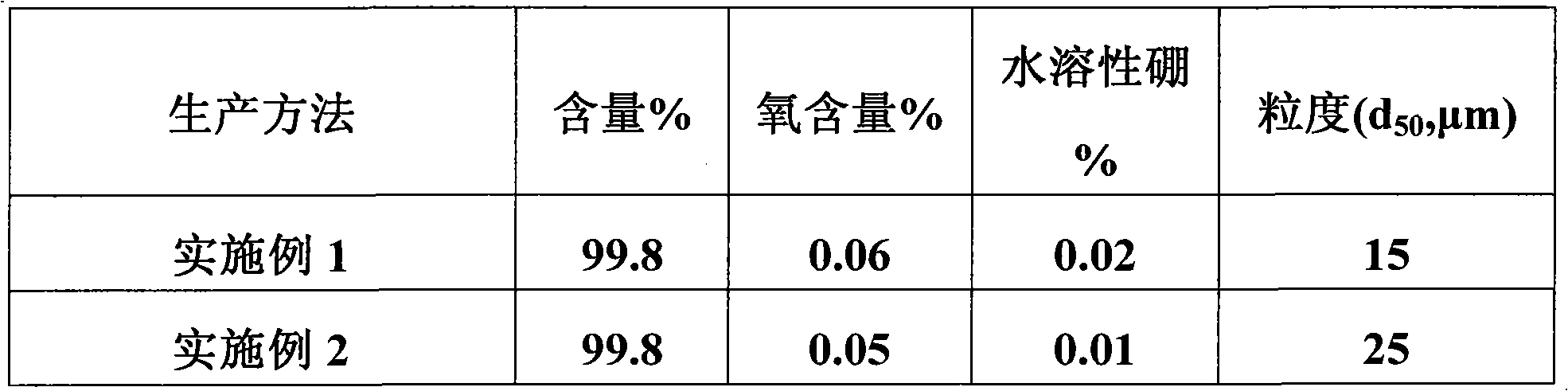
Embodiment 1
[0035] In the enamel kettle, add 600 kg of water, add 50 kg of boric acid, then add 48 kg of melamine in batches within 1 hour, react at 95°C for 2 hours, then drop to room temperature, filter, and dry;
[0036] Put the product obtained above into a crucible, put it into a high-temperature furnace, raise the temperature to 1600°C within 5 hours, and keep it warm for 3 hours;
[0037] The product obtained above was reacted with 4% hydrochloric acid at 95° C. for two hours, filtered and dried to obtain a large crystal hexagonal boron nitride powder product.
Embodiment 2
[0039] In the enamel reaction kettle, add 500 kg of the filtrate in Example 1, add 100 kg of water, add 50 kg of boric acid and 2 kg of barium carbonate, then add 48 kg of melamine in batches within 1 hour, and react at 95 ° C for 2 hours , then lowered to room temperature, filtered, and dried;
[0040] Put the product obtained above into a crucible, put it into a high-temperature furnace, raise the temperature to 1600°C within 5 hours, and keep it warm for 3 hours;
[0041] The product obtained above was reacted with 4% hydrochloric acid at 95° C. for two hours, filtered and dried to obtain a large crystal hexagonal boron nitride powder product.
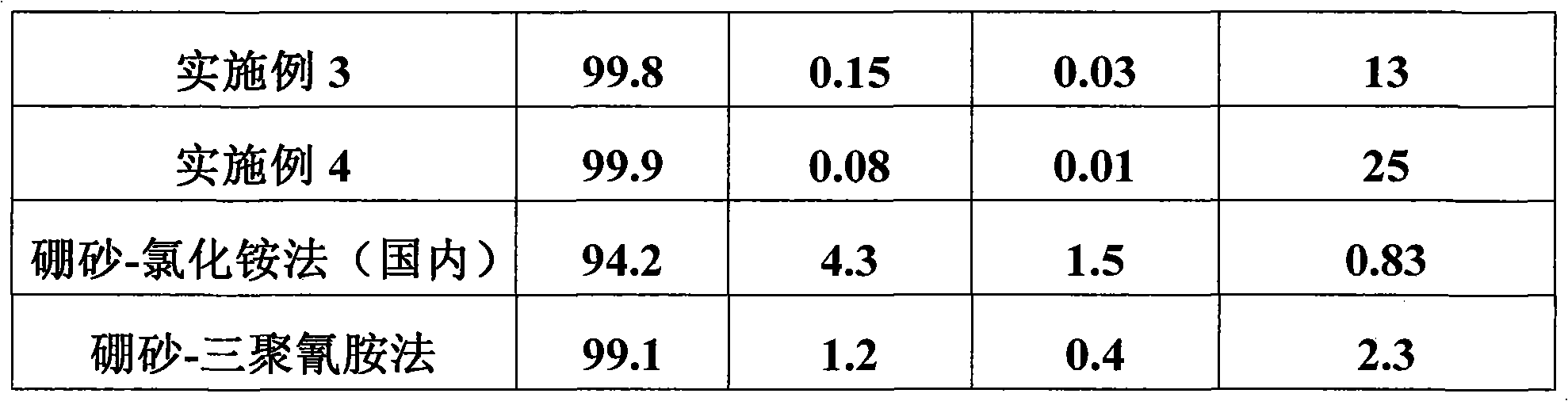
Embodiment 3
[0043] In the enamel kettle, add 600 liters of water, add 50 kg of boric acid, then add 51 kg of melamine in batches within 1 hour, react at 95 ° C for 2 hours, then drop to room temperature, filter, and dry;
[0044] Put the product obtained above into a ceramic plate, put it into a tunnel kiln furnace at 1400°C, and bake it for 11 hours;
[0045] The product obtained above was reacted with 4% hydrochloric acid at 95° C. for two hours, filtered and dried to obtain a large crystal hexagonal boron nitride powder product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com