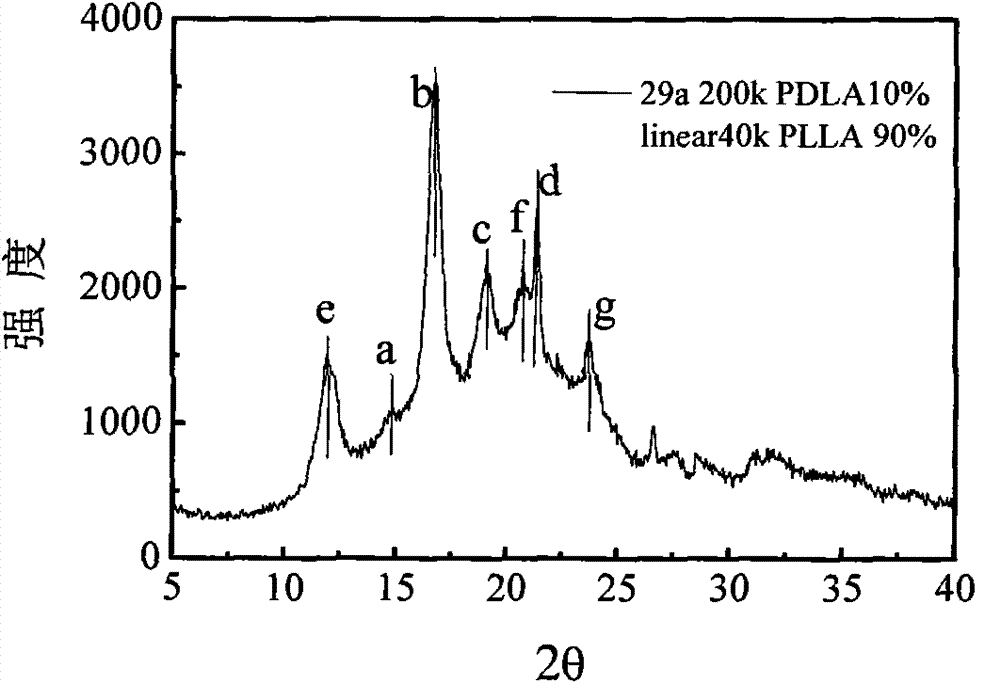
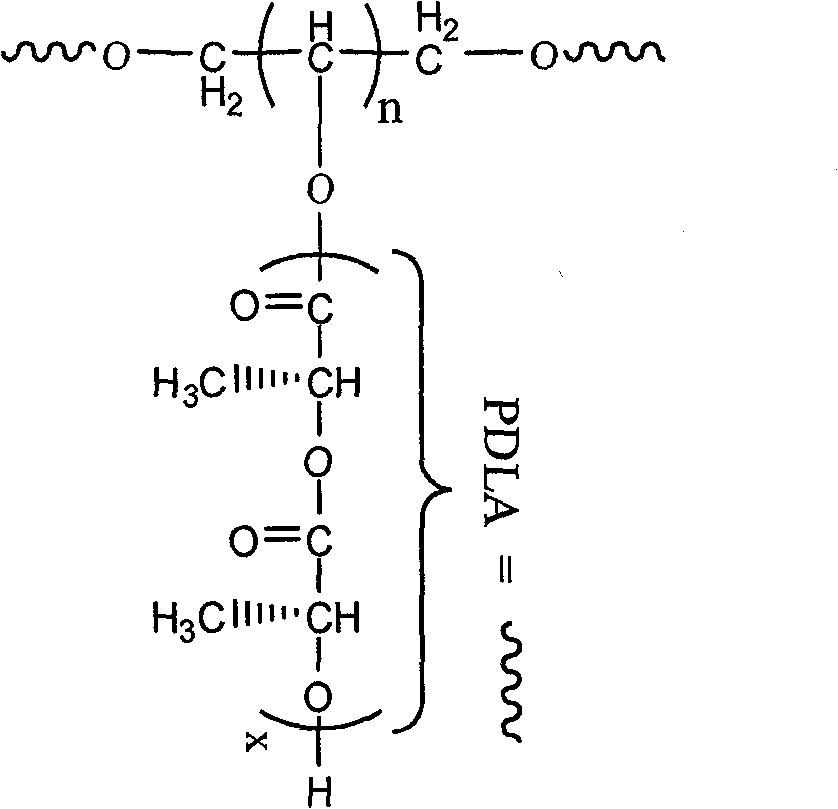
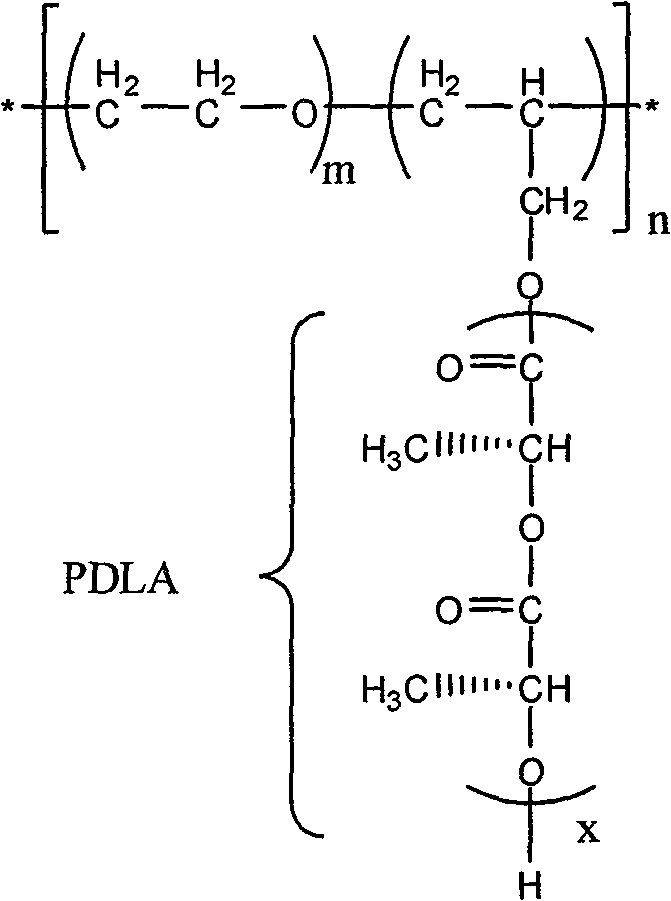
Modified polyactic acid and preparation method thereof
A technology of polylactic acid and dextrorotatory polylactic acid, which is applied in the field of modified polylactic acid and its preparation, can solve the problems of poor interaction, limiting the performance of PLA/montmorillonite composite materials, and difficulty in uniform dispersion of montmorillonite, and achieves phase Good capacity, improved strength and toughness, and high tensile elastic modulus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1. Preparation of modified polylactic acid by blending star-shaped multi-arm PDLA and linear PLLA
[0048] 1. Synthesis of linear PLLA matrix with a molecular weight of 40k
[0049] Under the protection of argon, add 0.0155g initiator ethylene glycol (Glycol), 10g L-lactide monomer (LLA), 0.012ml concentration in the dry 200ml reaction bottle successively and be 0.829mol / L stannous octoate ( SnOct 2 ) in toluene and 30ml of dry toluene. The reaction vial was sealed and placed in an oil bath at 120°C for 20 h. It was then dissolved in dichloromethane, precipitated with methanol, washed three times, and dried in vacuo to obtain linear PLLA.
[0050] The molecular weight and molecular weight distribution of the prepared linear PLLA are shown in Table 1.
[0051] 2. Synthesis of star-shaped six-arm branched PDLA (n=4) with a molecular weight of 400k
[0052] Under the protection of argon, add 0.0045g initiator sorbitol (sorbitol), 10g D-lactide monomer, 0.0036m...
Embodiment 2
[0057] Example 2, Modified polylactic acid prepared by blending comb-like multi-arm PDLA and linear PLLA
[0058] 1. Synthesis of comb-shaped twenty-nine-arm branched PDLA (n=29) with a molecular weight of 200k
[0059] (1) Macromolecular initiator-copolymer of polyethylene glycol and polyglycidyl alcohol containing twenty-nine hydroxyl groups (PEG 214 -co-PG 29 ) (synthetic method according to literature: Preparation of NovelPoly(ethylene oxide-co-glycidol)-graft-Poly(ε-caprolactone) Copolymers and Inclusion Complexation of the Grafted Chains with α-Cyclodextrin. Juan Huang, Zhongyu Li, Xuewei Xu, Yong Ren, Junlian Huang. Journal of Polymer Science: Part A: Polymer Chemistry, 2006, 44, 3684-3691).
[0060] ① Preparation of Vinyl Ether Glycidyl Alcohol:
[0061] Add 100mL ethyl vinyl ether and 25g glycidol to a dry and clean 500mL three-neck flask respectively. Dissolve 0.625 g of p-toluenesulfonic acid in 20 mL of diethyl ether, and add the diethyl ether solution dropwise...
Embodiment 3
[0073] Example 3, preparation of modified polylactic acid by blending star-shaped multi-arm block polylactic acid and linear PLLA
[0074] 1. Preparation of linear PLLA matrix with a molecular weight of 500k
[0075] Under the protection of argon, add 0.0012g initiator ethylene glycol (Glycol), 10g L-lactide monomer (LLA), 0.005ml concentration in the dry 200ml reaction bottle successively and be 0.829mol / L stannous octoate ( SnOct 2 ) in toluene and 30ml of dry toluene. The reaction vial was sealed and placed in an oil bath at 120° C. for 90 h. It was then dissolved in dichloromethane, precipitated with methanol, washed three times, and dried in vacuo to obtain linear PLLA.
[0076] The molecular weight and molecular weight distribution of the prepared linear PLLA are shown in Table 1.
[0077] 2. Synthesis of star-shaped five-arm block PDLA (5a-PL, DLA100k-b-PDLA100k)
[0078] The branched block copolymers were synthesized by a two-step addition method. Under the prote...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com