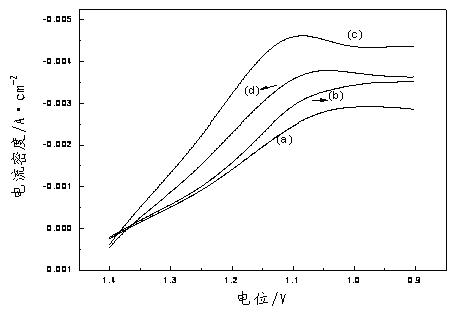
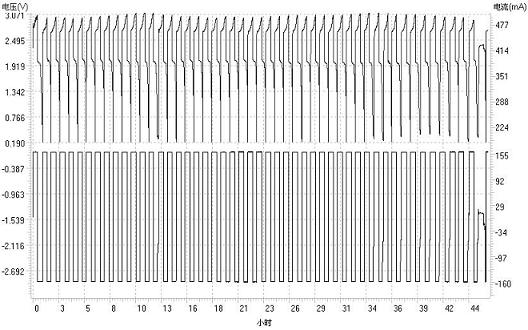
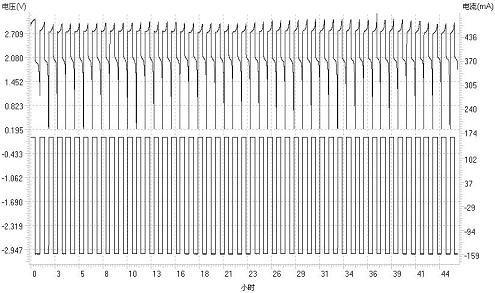
Cerium ion electrolyte using silver ion as anode catalyst and preparation method thereof
A technology of cerium ion and electrolyte, which is applied in the application field of Ag+ in the redox reaction of Ce3+/Ce4+ pair, can solve the problems of high voltage loss and low reaction rate, achieve good conductivity, simple preparation method, and improve circulation performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Weigh 28.79g of methanesulfonic acid and add it into 40mL of deionized water and stir evenly to form a methanesulfonic acid solution.
[0036] (2) Slowly add 22.11 g of cerium carbonate into the above methanesulfonic acid solution and stir evenly, and control the reaction temperature below 60°C.
[0037] (3) Weigh 10.00g of methanesulfonic acid and add it to 10mL of deionized water, then add 0.0276g of silver carbonate, and stir evenly to obtain a silver carbonate solution. Cool the solution obtained in step (2) to room temperature and add it to the above-mentioned silver carbonate solution, then add deionized water to make the volume to 100mL to obtain Ag + The concentration is 0.001mol×L -1 , a cerium methanesulfonate electrolyte with a cerium ion concentration of 1mol / L.
[0038] The resulting electrolyte is used as the positive electrolyte, and the negative electrolyte uses ZnSO 4 solution, assembled into a Zn-Ce battery. The positive and negative electrodes...
Embodiment 2
[0040] (1) Weigh 28.79g of methanesulfonic acid and add it into 30mL of deionized water and stir evenly to form a methanesulfonic acid solution.
[0041] (2) Slowly add 22.11 g of cerium carbonate into the above methanesulfonic acid solution and stir evenly, and control the reaction temperature below 60°C.
[0042] (3) Weigh 10.00 g of methanesulfonic acid and add it to 10 mL of deionized water, then add 0.1656 g of silver carbonate, and stir evenly to obtain a silver carbonate solution. Cool the solution obtained in step (2) to room temperature and add it to the above-mentioned silver carbonate solution, then add deionized water to make the volume to 100mL to obtain Ag + The concentration is 0.006mol×L -1 , a cerium methanesulfonate electrolyte with a cerium ion concentration of 1mol / L.
[0043] The resulting electrolyte is used as the positive electrolyte, and the negative electrolyte uses ZnSO 4 solution, assembled into a Zn-Ce battery. The positive and negative electrode...
Embodiment 3
[0045] (1) Weigh 28.79g of methanesulfonic acid and add it into 30mL of deionized water and stir evenly to form a methanesulfonic acid solution.
[0046] (2) Slowly add 22.11 g of cerium carbonate into the above methanesulfonic acid solution and stir evenly, and control the reaction temperature below 60°C.
[0047] (3) Weigh 10.00 g of methanesulfonic acid and add it to 10 mL of deionized water, then add 0.276 g of silver carbonate, and stir evenly to obtain a silver carbonate solution. Cool the solution obtained in step (2) to room temperature and add it to the above-mentioned silver carbonate solution, then add deionized water to make the volume to 100mL to obtain Ag + The concentration is 0.01mol×L -1 , a cerium methanesulfonate electrolyte with a cerium ion concentration of 1mol / L.
[0048] The resulting electrolyte is used as the positive electrolyte, and the negative electrolyte uses ZnSO 4 solution, assembled into a Zn-Ce battery. The positive and negative electrode...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com