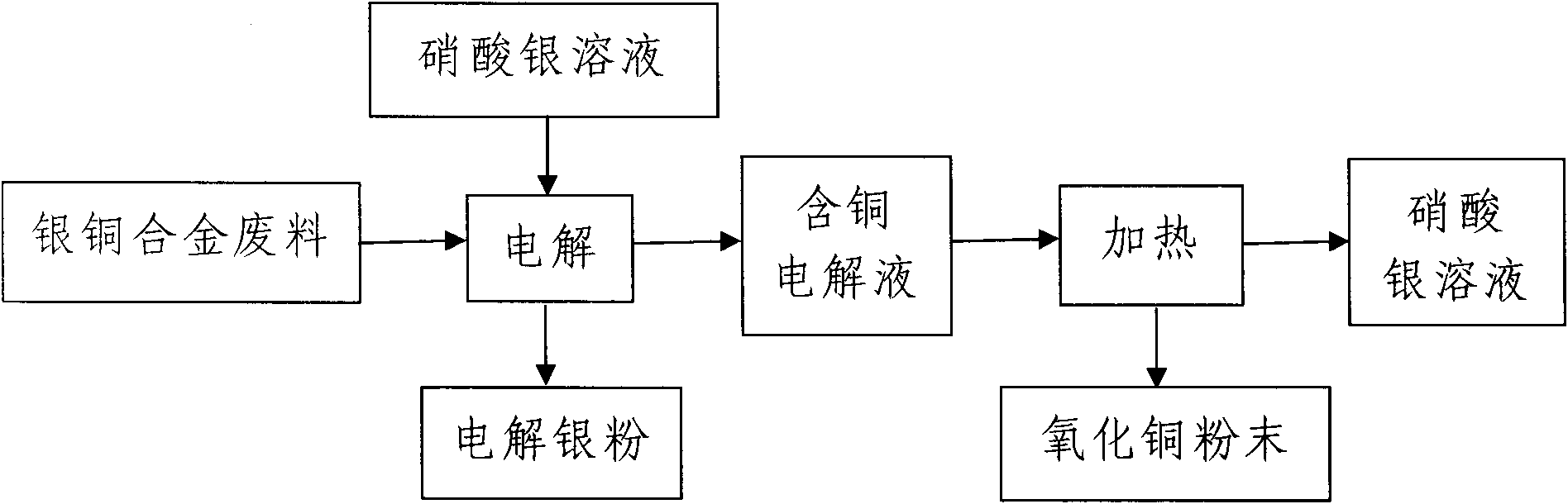
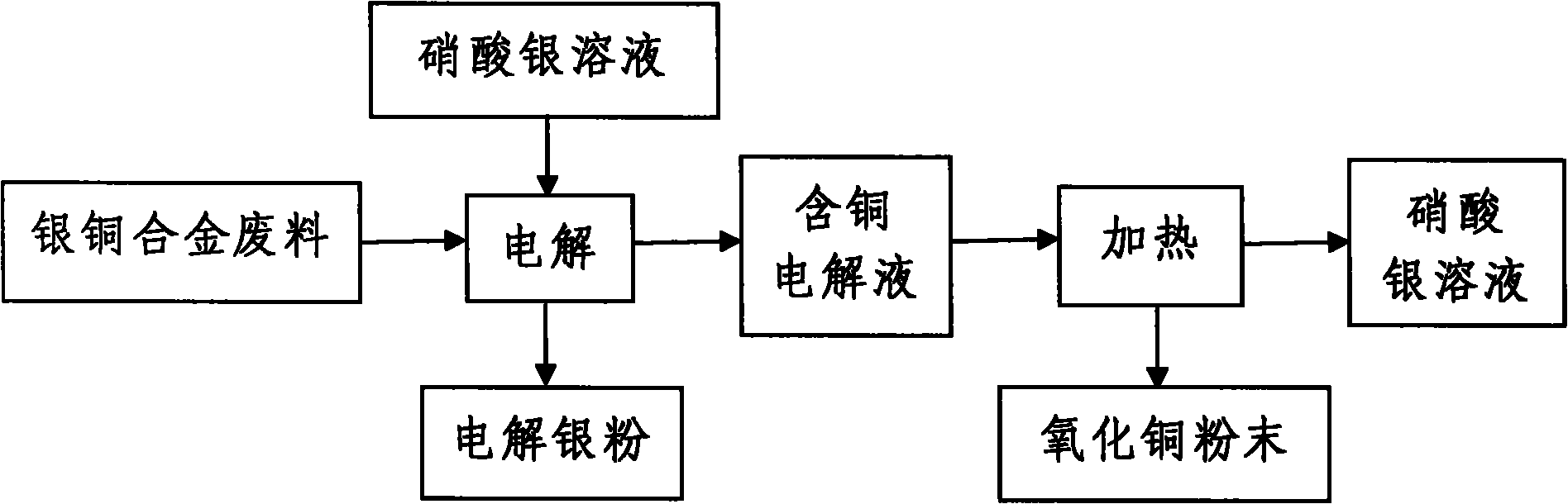
Method for recycling silver and copper from silver-copper alloy scrap
A silver-copper alloy and waste technology, applied in the field of alloy waste recycling, can solve the problems of a large amount of flue gas, waste liquid, high consumption of reagents and high processing cost, and achieve the effects of less environmental pollution, high product quality and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] (1) The silver nitrate solution with a silver ion concentration of 180g / L is used as the electrolyte, the electrolyte is loaded into the electrolytic cell, the titanium plate is used as the cathode, and the AgCu 10 Alloy scrap loaded into titanium anode basket as anode, AgCu 10 The amount of alloy waste added is 10Kg / 50L electrolyte, and the current density at the cathode is 200A / m 2 , the cell voltage is 2.2V under the condition of electrification electrolysis;
[0018] (2) Add an appropriate amount of AgCu to the titanium anode basket every 6 hours during the electrolysis process 10 Alloy Scrap, AgCu 10 The copper in the alloy waste continuously enters the electrolyte in the form of ions to obtain a copper-containing electrolyte. Every 12 hours, a titrator is used to analyze the concentration of silver ions and copper ions in the copper-containing electrolyte, and a pH meter is used to analyze the pH value of the copper-containing electrolyte. It is 180g / L to contr...
Embodiment 2
[0022] (1) The silver nitrate solution with a silver ion concentration of 200g / L is used as the electrolyte, the electrolyte is loaded into the electrolytic cell, the titanium plate is used as the cathode, and the AgCu 20 Alloy scrap loaded into titanium anode basket as anode, AgCu 20 The amount of alloy waste added is 60Kg / 50L electrolyte, and the current density at the cathode is 400A / m 2 , the cell voltage is 2.5V under the condition of electrification electrolysis;
[0023] (2) Add an appropriate amount of AgCu to the titanium anode basket every 24 hours during the electrolysis process 20 Alloy Scrap, AgCu 20 The copper in the alloy waste continuously enters the electrolyte in the form of ions to obtain a copper-containing electrolyte. Every 72 hours, a titrator is used to analyze the concentration of silver ions and copper ions in the copper-containing electrolyte, and a pH meter is used to analyze the pH value of the copper-containing electrolyte. It is 200g / L to cont...
Embodiment 3
[0027] (1) The silver nitrate solution with a silver ion concentration of 190g / L is used as the electrolyte, the electrolyte is loaded into the electrolytic cell, the titanium plate is used as the cathode, and the AgCu 28 Alloy scrap loaded into titanium anode basket as anode, AgCu 28 The amount of alloy waste added is 50Kg / 50L electrolyte, and the current density at the cathode is 300A / m 2 , the cell voltage is 2.35V under the condition of energized electrolysis;
[0028] (2) Add an appropriate amount of AgCu to the titanium anode basket every 15 hours during the electrolysis process 28 Alloy Scrap, AgCu 28 The copper in the alloy waste continuously enters the electrolyte in the form of ions to obtain a copper-containing electrolyte. Every 42 hours, a titrator is used to analyze the concentration of silver ions and copper ions in the copper-containing electrolyte, and a pH meter is used to analyze the pH value of the copper-containing electrolyte. It is 190g / L to control t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com