Medicament-carrying lipoid particulates and preparation method thereof
A particle and drug-carrying technology, applied in pharmaceutical formulations, microcapsules, drug delivery, etc., can solve the problems affecting the effectiveness, safety and stability of preparations, storage, transportation difficulties, and short action time, etc., to prolong the action time of drugs, improve Drug encapsulation efficiency, the effect of increasing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: tea polyphenol lipid particle suspension
[0028] Many drugs are sensitive to light and heat conditions, and they are easy to lose their efficacy when placed in the environment. For example, tea polyphenols are polyphenolic substances extracted from tea leaves, which have good anti-mutation, anti-tumor, anti-oxidation, anti-allergic, anti-caries and other pharmacological effects, but they are sensitive to heat and easily oxidized. Tricky issues in production. In the first embodiment of the present invention, tea polyphenols are used as target drugs, and cetyl alcohol is used as lipid material and medium molecular weight hydroxyethyl starch to prepare tea polyphenol lipid particles.
[0029] Preparation of tea polyphenol lipid particle suspension: 0.5g hydroxyethyl starch 130 / 0.4 and 5mg Tween 80 were dissolved in 25ml distilled water to form the water phase, 10mg tea polyphenols and 15mg cetyl alcohol were dissolved in 20ml absolute ethanol In order to f...
Embodiment 2
[0036] Embodiment 2: Freeze-dried product of tea polyphenol lipid microparticles
[0037] The dried lipid particles are convenient for storage and transportation, and have improved thermal stability. The second embodiment of the present invention is to make the tea polyphenol lipid microparticles obtained in the first embodiment into a solid freeze-dried product by using a freeze-drying process.
[0038] Preparation of tea polyphenol lipid microparticle freeze-dried product: the suspension of tea polyphenol lipid microparticle A and tea polyphenol lipid microparticle B obtained in the first embodiment was added with 50 mg trehalose as a freeze-drying support agent, Transfer to a jar, freeze at -20°C for 1 hour, and freeze-dry (5×10-4Pa, 20 hours) to obtain freeze-dried powder of tea polyphenol lipid particle A and tea polyphenol lipid particle B. The same method was used to prepare the freeze-dried powder of blank lipid microparticles without tea polyphenols.
[0039]Determi...
Embodiment 3
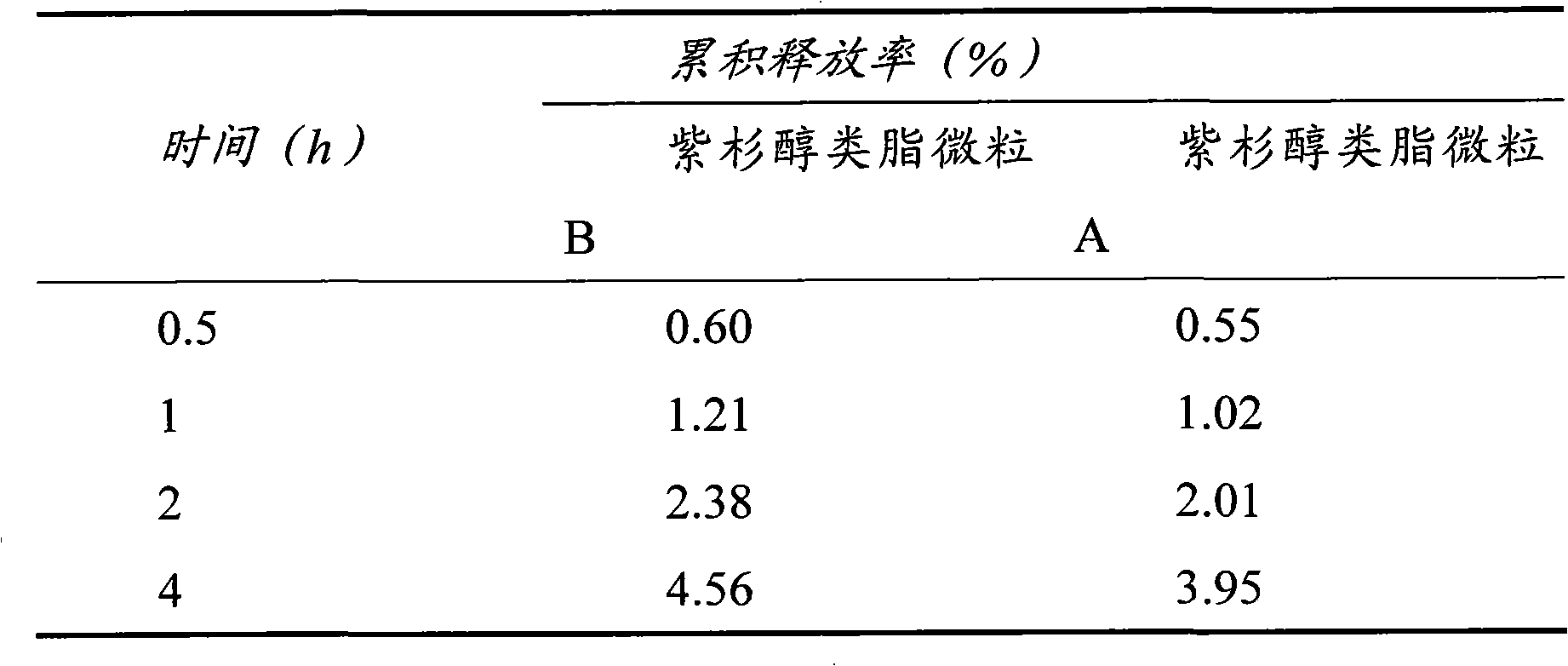
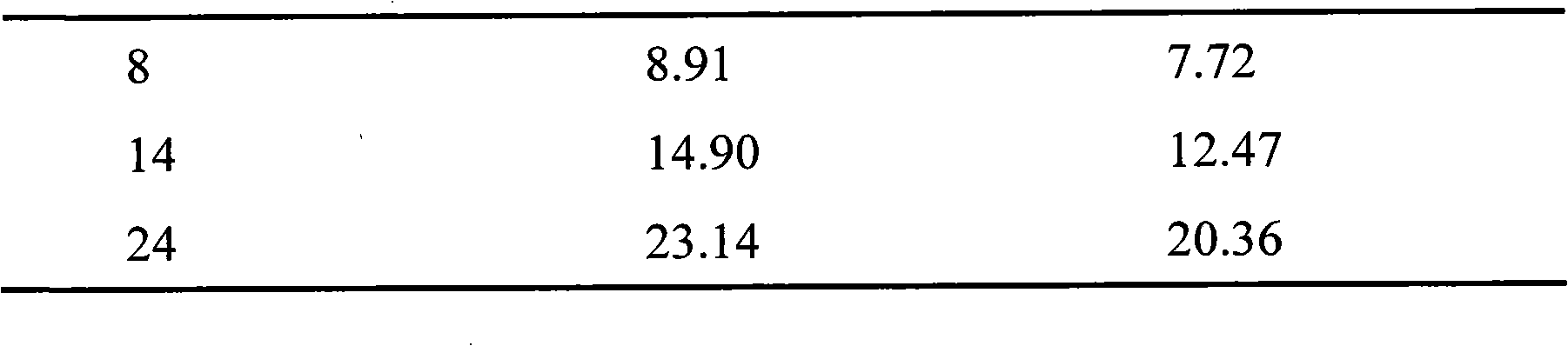
[0044] Example 3: Paclitaxel Lipid Microparticles
[0045] Many drugs need to be long-acting to reduce the frequency of dosing. In the third embodiment of the present invention, the antineoplastic drug paclitaxel is used as the target drug, and stearic acid is used as the lipid material and hydroxyethyl starch with high molecular weight and high substitution level to prepare long-acting paclitaxel lipid particles.
[0046] Preparation of paclitaxel lipid microparticles: 10 mg of paclitaxel, 100 mg of stearic acid and 10 ml of acetone were added into a 25 ml stoppered pear-shaped bottle, and ultrasonically dissolved to form an organic phase. Another 150 mg of poloxamer 188 was dissolved in 30 ml of water to form the water phase. Inject the organic phase into the water phase at 70°C stirred at 1000r / min, continue to stir for 4h, evaporate the acetone completely and concentrate the system to about 5ml to form a paclitaxel lipid microparticle suspension, which is divided into two...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com