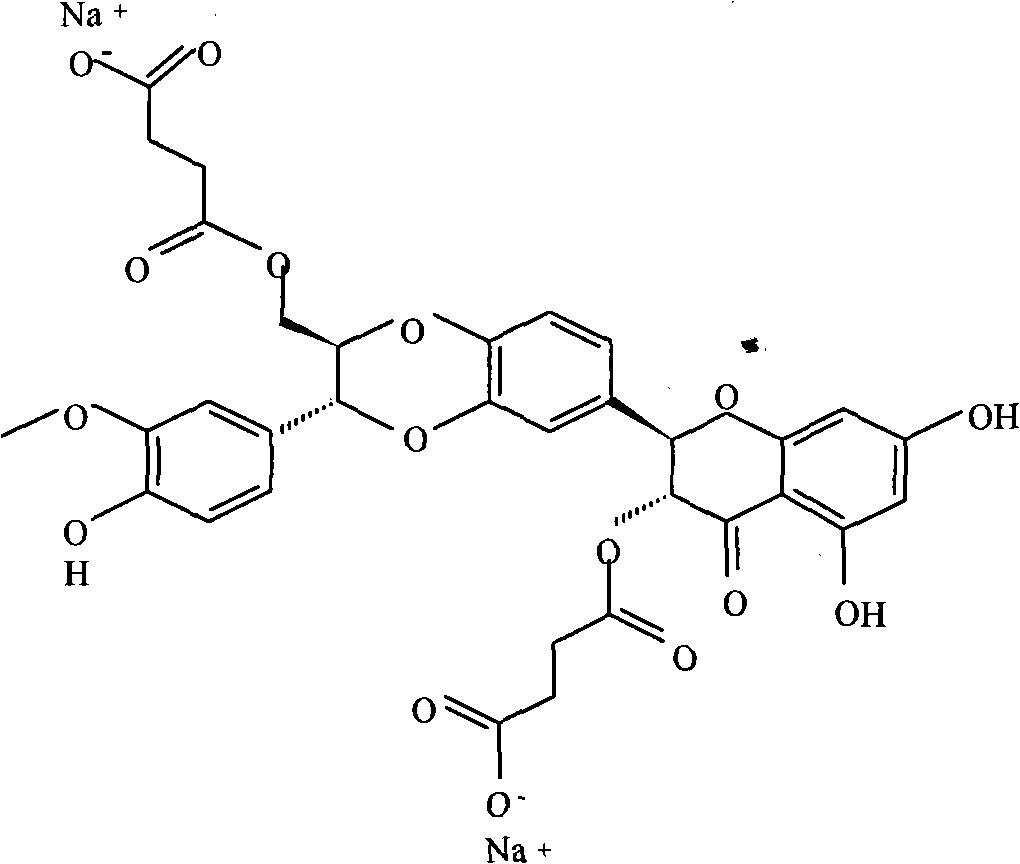
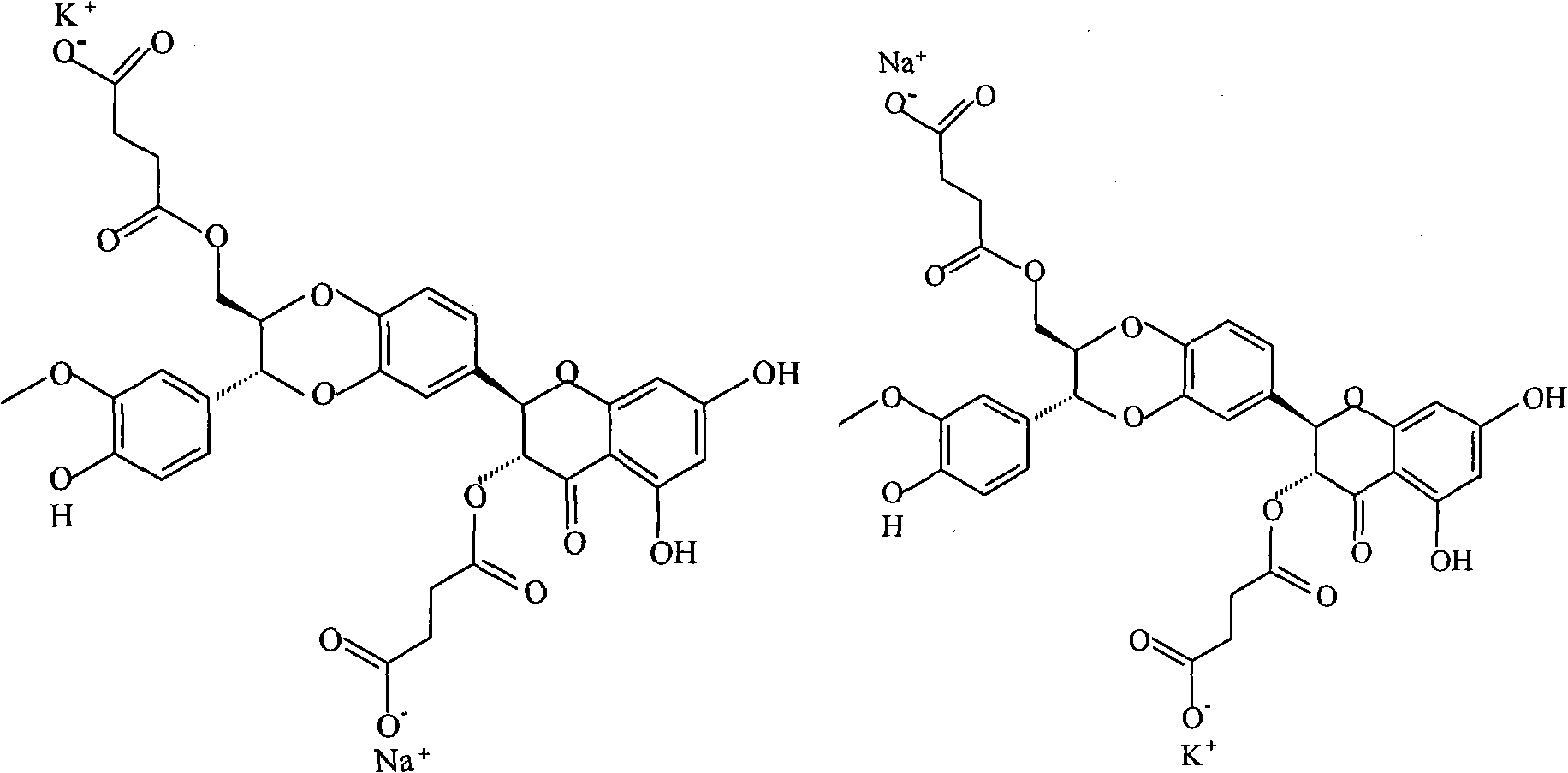
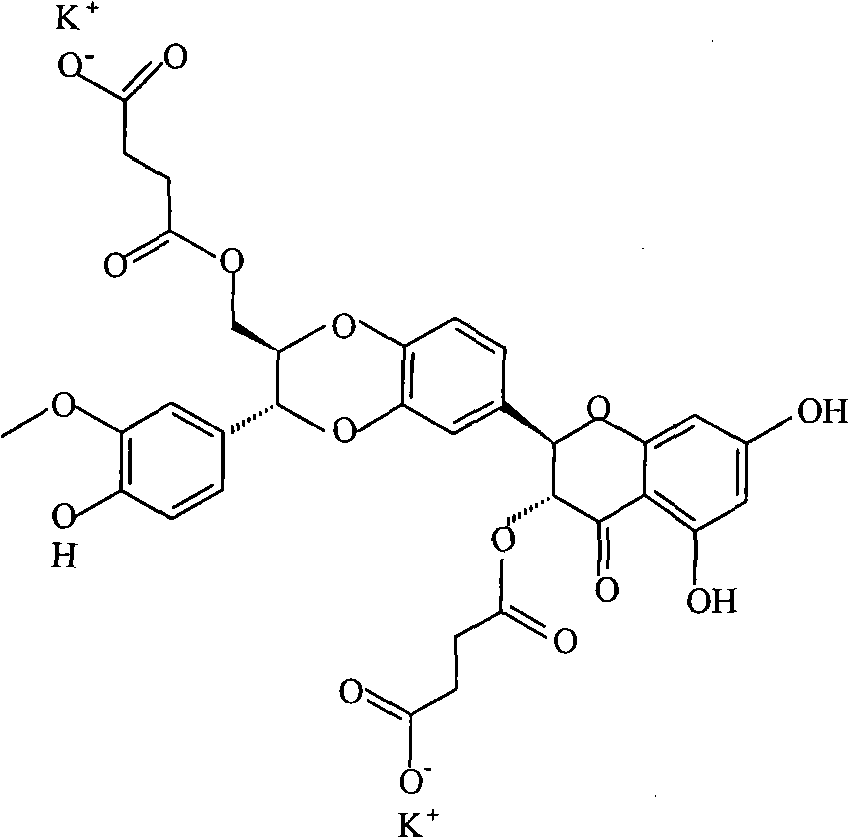
Phospholipid complex of silybin dihemisuccinate disodium and preparation method and application thereof
A dimesuccinate and phospholipid complex technology, applied in the field of medicine, can solve the problems of low bioavailability and poor water solubility, and achieve the effect of expanding the scope of application, increasing fat solubility, and expanding the route of drug delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 silibinin succinate disodium salt phospholipid complex
[0037] Weigh 530.0 g of silibinin succinate disodium salt and mix them with 1000.0 g of soybean lecithin, dissolve in 20000 ml of methanol, heat at 50°C, the solution is clear after 1h, concentrate and dry under reduced pressure at 75°C to obtain silibinin succinate di Sodium Salt Phospholipid Complex.
Embodiment 2
[0038] Example 2 Preparation of silibinin succinate disodium salt phospholipid complex compressed tablet
[0039] Mix 530.0g of silibinin succinate disodium salt and 500.0g of soybean lecithin, dissolve in 20000ml of absolute ethanol, heat at 50°C, the solution is clear after 1h, concentrate under reduced pressure at 70°C until viscous, add microcrystalline 18.0g of cellulose, 450.0g of lactose, 180g of pregelatinized starch, and 22.5g of crospovidone are mixed evenly, and the aqueous solution containing 15% of povidone is used as a wetting agent to make a soft material, granulated with a 20-mesh sieve, 55°C Ventilate and dry for 3 hours, granulate, add 13.5.0 g of talcum powder and 13.5.0 g of magnesium stearate, mix and press to obtain about 10,000 tablets, each containing 35 mg of silibinin, to obtain compressed tablets.
Embodiment 3
[0040] Example 3 Preparation of silibinin succinate disodium salt phospholipid complex capsule
[0041] Mix 530.0g of silibinin succinate disodium salt and 1000.0g of soybean lecithin, dissolve in 20000ml of absolute ethanol, heat at 50°C, the solution is clear after 1h, concentrate under reduced pressure at 70°C until viscous, add lactose 450.0 g, 360.0g of mannitol, 72g of carboxymethyl starch sodium, put into the fast stirring granulator KJZ-IO, spray 30% ethanol aqueous solution to granulate, ventilate and dry at 60°C for 3 hours, granulate with 20 mesh sieve, add stearic acid Magnesium 27.0g is mixed evenly, and No. 1 gelatin hard capsule is filled, so that each capsule contains silybin 35mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com