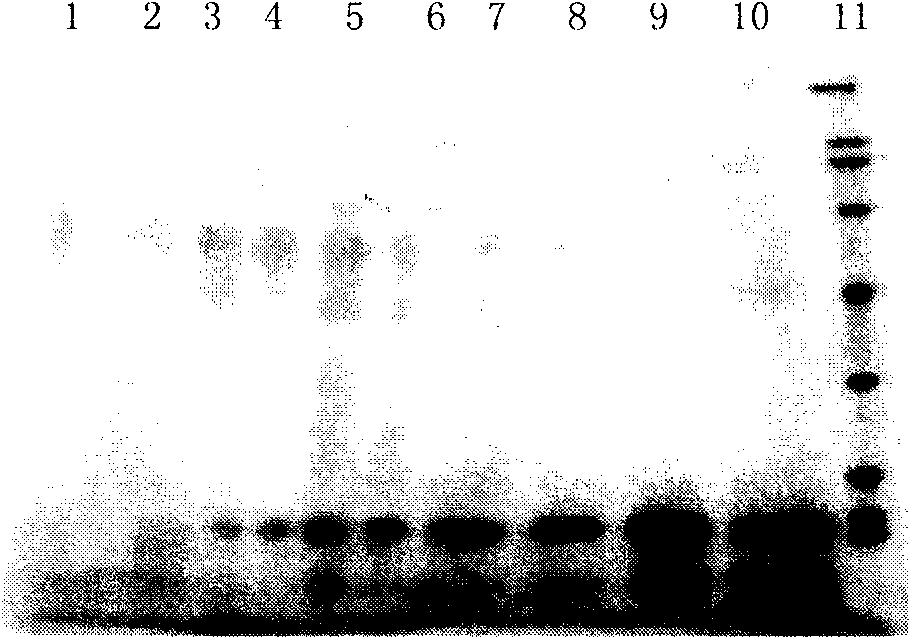Preparation of new apolipoprotein A-II
A technology of apolipoprotein and A-II, which is applied in the preparation method of apolipoprotein and peptide, animal/human protein, etc., to achieve the effects of low production cost, stable expression system and stable strain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Cloning and amplification of human apolipoprotein A-II gene (hApoA-II)
[0043] (1) Trizol method to extract total RNA from human liver tissue
[0044] The freshly isolated human liver tissue was cut into 100 mg size and immediately frozen in liquid nitrogen. Total RNA was extracted from tissues as follows. Take out 300 mg of frozen tissue and put it into a mortar filled with liquid nitrogen, and crush the tissue. Transfer the crushed tissue into a 50ml centrifuge tube, add about 5ml Trizol, and homogenize with a homogenizer at high speed for 15-30s at room temperature. Add 1.0ml of chloroform (200μl / ml Trizol), vortex fully, and place at room temperature for 5min. After centrifugation (12000r / min) at 4°C for 15min, the upper aqueous phase was transferred to another centrifuge tube. Add an equal volume of isopropanol, shake well, precipitate at room temperature for 10 minutes, centrifuge at 4°C (12000r / min) for 15 minutes, and discard the supernatant. Ad...
Embodiment 2
[0080] Example 2: Construction of hApoA-II Pichia secretory expression vector pPICZa / hApoA-II and host cell transformation
[0081] Take 50ng of the plasmid carrying the hApoA-II gene correctly identified by the above sequencing, and set up a PCR reaction system in a 0.2ml EP tube according to the above ratio. Using the above PCR reaction system and conditions, with the help of the upstream primer 5'-AACCTCGAGAAGAGACAGGCAAAGGAGCCATGT-3' (SEQ ID NO: 3) to introduce the partial sequence of the yeast a factor signal peptide and the XhoI site, and using the downstream primer: 5'-GCGTCTAGATCACTGGGTGTTGAGCTTCTTAG-3 '(SEQ ID NO: 4) introduces an EcoR I site. After introducing the above sequences and restriction sites by PCR, recover the target fragment. After cutting with the corresponding enzymes, the plasmid pPICZa cut with the same enzymes was connected to construct the Pichia pastoris expression vector pPICZa / hApoA-II, and then transformed into Escherichia coli, the plasmid was ...
Embodiment 3
[0086] Embodiment 3: Expression and purification of ApoA-II polypeptide
[0087] (1) Expression of recombinant human apolipoprotein A-II (rhApoA-II)
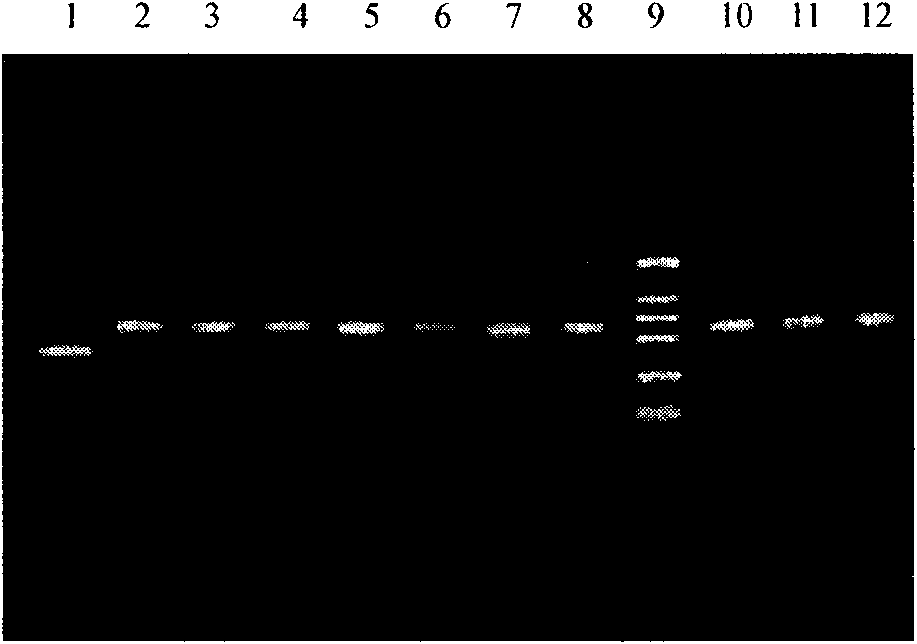
[0088] Inoculate 10 ml of BMGY (pH 6.0) medium with positive clones from the above identification results, and incubate with shaking at 30°C for 24 hours until OD 600 Cells were collected when reaching 2.0-6.0. Resuspend the cell pellet with an equal volume (10ml) of BMMY (pH6.0), culture with shaking at 30°C, and induce expression. During the induction process, methanol was supplemented every 24 hours to a final concentration of 0.5%, and sterilized ultrapure water was supplemented at the same time to keep the total volume of the fermentation broth constant. At the 0, 24, 48, 72, 96, 120, 144, and 168 hours of culture, take 0.5ml of fermentation broth, centrifuge and take the supernatant for protein analysis (SDS-PAGE, N-terminal amino acid sequence analysis, etc.) ).
[0089] (2) Purification of rhApoA-II
[0090] The fer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com