Double-phenothiazine-based dye, and preparation method and application thereof
A phenothiazine-based dye technology, which can be used in thiazine dyes, methine-based/polymethine-based dyes, organic dyes, etc., can solve the difficulty of separation and purification of ruthenium polypyridine complexes, reduce large-scale practical application, and limit the cost of DSSCs And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Synthesis of 1,6-bis-(3-cyanoacrylate phenothiazinyl)-n-hexane
[0071] All reactions were carried out in a dry environment under nitrogen protection
[0072] (1) Synthesis of 1,6-bisphenothiazinyl n-hexane
[0073]
[0074] Dissolve 1.99g (10mmol) of phenothiazine in 15ml of DMSO, add 2.24g (40mmol) of KOH, and vigorously stir at room temperature for 20min. Then 1.22g (5mmol) of 1,6-dibromohexane was added dropwise, and the reaction was continued for 48h. After the reaction was completed, it was poured into an ice-water mixture and stirred vigorously for 30 min, filtered, and the solid was dissolved in dichloromethane, washed 3 times with water, and the organic phase was dried, and the crude product was recrystallized to obtain 2.01 g (4.18 mmol) of the product, with a yield of 83.6%. . Melting point: 163-165°C.
[0075] (2) Synthesis of 1,6-bis-(3-formylphenothiazinyl)-n-hexane
[0076]
[0077] Add 1.23g (8mmol) POCl to 1-20 parts of 731mg (10mmol) DMF in ...
Embodiment 2
[0082] Synthesis of N-ethyl-3-cyanoacrylate-phenothiazine
[0083] All reactions were carried out in a dry environment under nitrogen protection
[0084] (1) Synthesis of N-ethylphenothiazine
[0085]
[0086]Dissolve 1.99g (10mmol) of phenothiazine in 15ml of DMSO, add 2.24g (40mmol) of KOH, and stir at room temperature for 20min. Then 1.64g (15mmol) of bromoethane was added dropwise, and the reaction was continued for 48h. Pour into ice-water mixture and stir for 10 min, filter, dissolve the solid in dichloromethane, wash 3 times with water, dry the organic phase, and recrystallize the crude product to obtain 1.70 g (7.48 mmol) product with a yield of 75%. Melting point: 104-105°C.
[0087] (2) Synthesis of N-ethyl-3-formyl-phenothiazine
[0088]
[0089] Add 1.23g (8mmol) POCl to 731mg (10mmol) DMF of 1-20 parts in Bingyu 3 , and then remove the ice bath, return to room temperature, and stir the reaction for 1h. Then, 455 mg (2 mmol) of N-ethylphenothiazine diss...
Embodiment 3
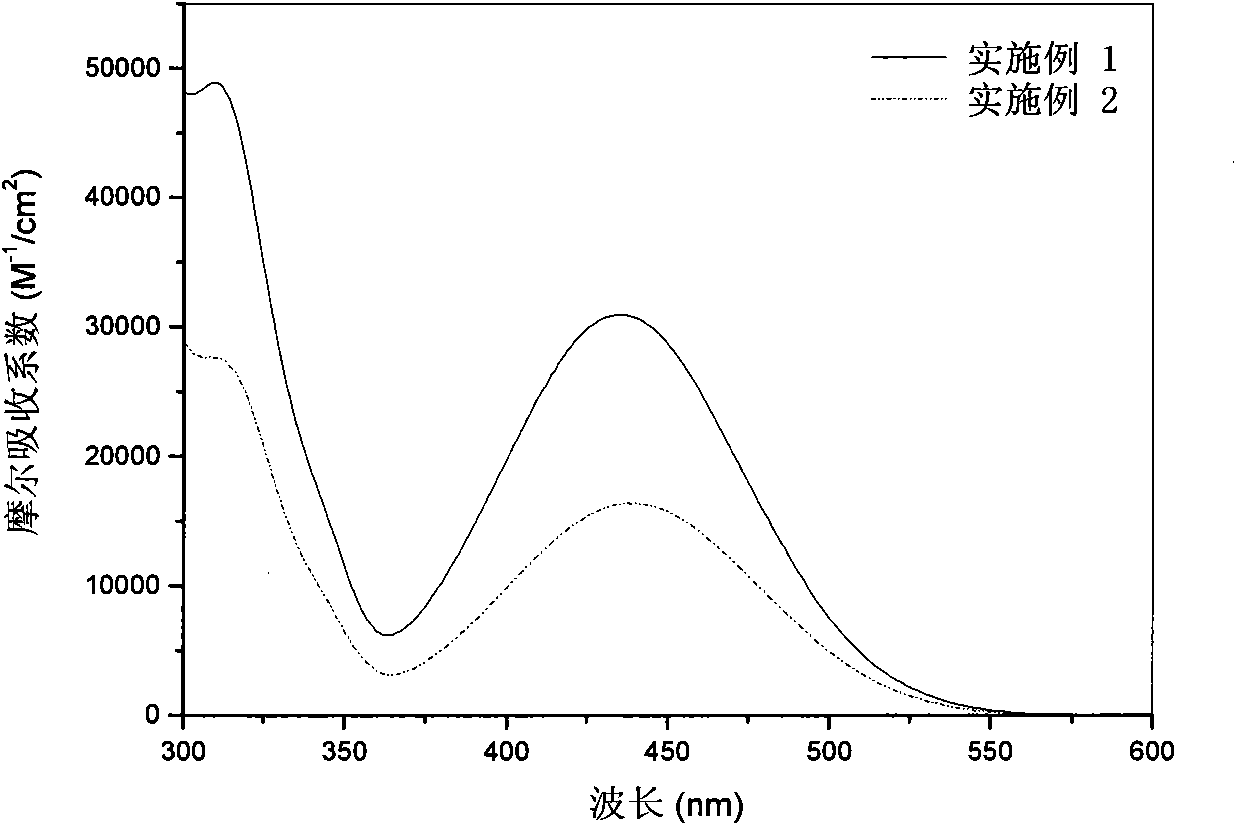
[0094] The ultraviolet-visible absorption spectrum / fluorescence spectrum test of embodiment 1 and embodiment 2 dyestuffs, ultraviolet-visible absorption spectrum and fluorescence emission spectrogram are respectively image 3 , Figure 4 shown.
[0095] Solvent: THF
[0096] Concentration: 2×10 -5 m
[0097] Temperature: room temperature
[0098] Instruments: Shimadzu UV-2450 UV-visible scenery photometer, Hitachi F-4500 fluorescence spectrometer
[0099] The maximum ultraviolet / visible absorption wavelength and the maximum fluorescence emission wavelength (nm) data comparison of the dyestuff in the embodiment 1 and 2 of table 1, the data obtained are summarized in table 1
[0100] Table 1
[0101] dye
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com