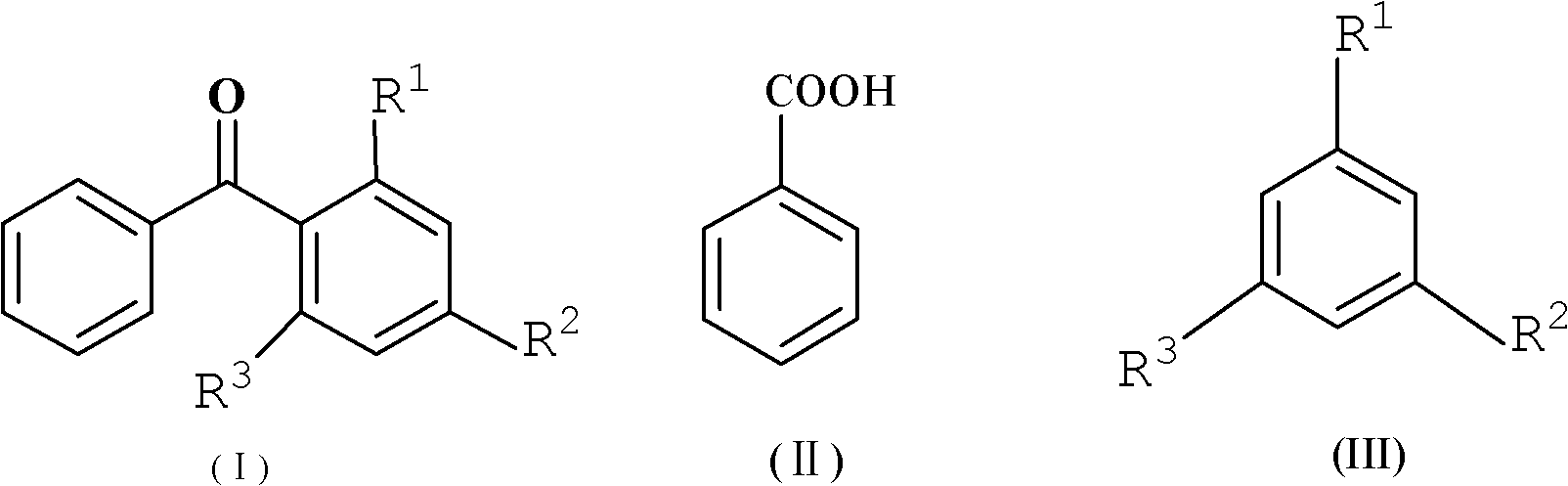
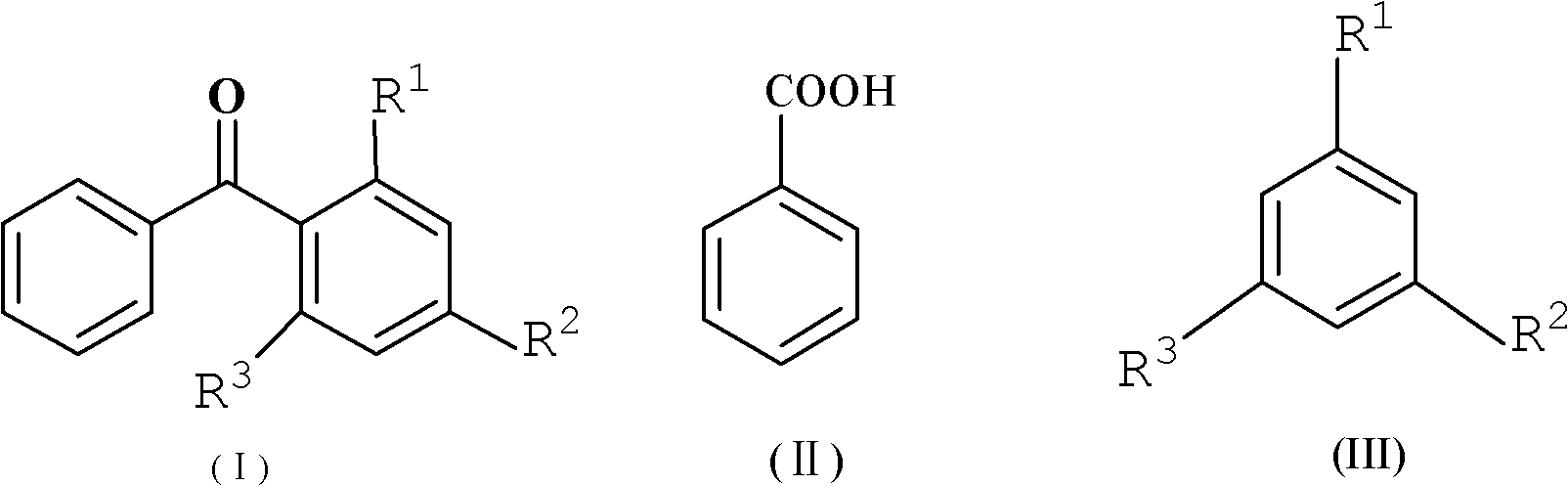
Method for synthesizing diaryl ketone compound
A diaryl ketone and synthesis method technology, which is applied in the field of catalytic substitution of benzene and benzoic acid to synthesize diaryl ketone compounds, can solve the problem of difficult control of active component load, large difference in catalytic activity, and target product yield. No high-level problems, easy to handle and store, easy post-processing, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Catalyst P 2 o 5 / SiO 2 preparation of
[0026] Commercially available 200-mesh silica gel was taken and dried at a constant temperature at 120°C for 24 hours in advance. Weigh 10g of silica gel in a 100mL round-bottomed flask, quickly weigh 10g of phosphorus pentoxide and mix it with silica gel, and mix well with a glass rod. After 5 minutes, a uniform white powder is obtained. Activate for 1 hour under the condition of heating in a furnace at 120°C, and finally get The P 2 o 5 / SiO 2 Catalyst, Catalyst is stored in a sealed dry glass bottle.
Embodiment 2
[0027] Example 2: Catalyst P 2 o 5 / Al 2 o 3 preparation of
[0028] Commercially available Al 2 o 3 , screen 100 meshes and dry at 120°C for 24 hours in advance, weigh 10g into a 100mL round bottom flask, quickly weigh 10g of phosphorus pentoxide and mix it with silica gel, mix well with a glass rod, and get a uniform white powder after 5 minutes. After activating for 1 hour at 120°C furnace temperature, the P 2 o 5 / Al 2 o 3 Catalyst, Catalyst is stored in a sealed dry glass bottle.
Embodiment 3
[0029] Embodiment 3: the preparation of 4-methylbenzophenone
[0030] Add benzoic acid (1.22g, 0.01mol), toluene (32.8mL, 0.3mol) and P 2 o 5 / SiO 2 Catalyst (4g), under the condition of reflux at 110 ℃, TLC tracking detection until the reaction is complete, the reaction time is 3 hours, after cooling to room temperature, suction filtration, recovery catalyst, the filtrate is extracted and separated with a separatory funnel, the organic layer is separated, added to qs. 10% (w / w) NaHCO 3 solution, filtered, and the organic layer was washed with anhydrous Na 2 SO 4 Drying, solvent removal under reduced pressure, obtain white crystal, obtain needle-like solid 1.39g after 10% (v / v) aqueous ethanol recrystallization, yield (in benzoic acid) is 71%, melting point 55-57 ℃ ( Literature value 53-57°C).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com