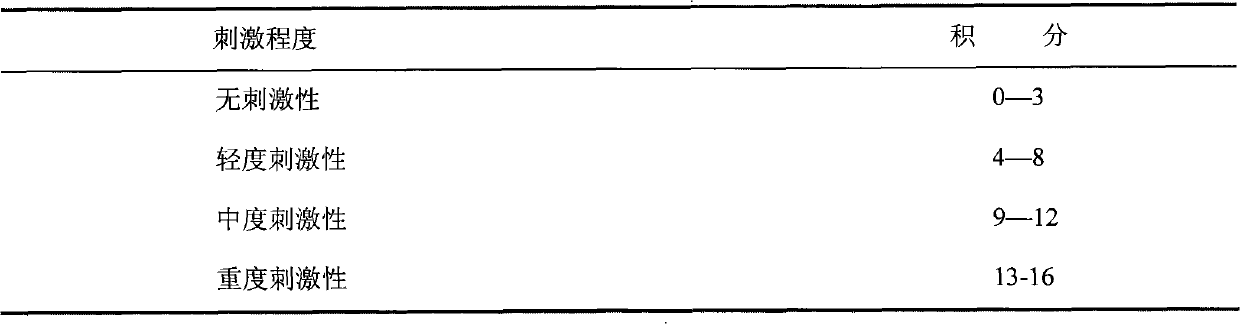
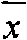Applications of human placenta peptide in preparing drugs for treating dry eye syndrome and other ocular surface diseases
A technology for human placenta and dry eye syndrome, applied in sensory diseases, drug combinations, pharmaceutical formulas, etc., can solve problems that are difficult to cure, have low curative effect, affect people's vision and quality of life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0071] 1. Preparation of human placental peptide stock solution:
[0072] Select healthy maternal placenta that is negative for HIV, hepatitis B virus, hepatitis C virus, and syphilis in prenatal serological tests. The placenta of pregnant women suffering from infectious hepatitis, AIDS, syphilis and other diseases, as well as the placenta of artificial abortion, stillbirth, and various deformities, are not used. The placenta is immediately brought back on ice under aseptic conditions after delivery. The placenta can also be refrigerated below -10°C. It should also be refrigerated during collection and transportation. If the placenta is frozen below -30°C, it should be 3 Applied within months. Take the placenta cord blood for HIV, hepatitis B virus, hepatitis C virus, syphilis, etc. The placenta with negative results can be selected and used.
[0073] Wash the brought back fresh placenta twice with pre-cooled medical physiological saline on the ultra-clean table according to the ...
Embodiment 1、50
[0077] Example 1. Preparation of 50% (volume fraction) human placental peptide eye drops
[0078] Human placental peptide stock solution 500ml
[0080] 1mol / L sodium hydroxide appropriate amount
[0081] Add water for injection to 1000ml
[0082] Take 4g of sodium chloride and add appropriate amount of water for injection to dissolve it, stir well, adjust the pH to 7.2±0.2 with 1mol / L sodium hydroxide, then add 500ml of human placental peptide stock solution, and then add water for injection to 1000ml. 0.2μm filter membrane filter sterilization, aseptic packaging, each 0.5ml.
[0083] 2. Preparation of thickening human placental peptide eye drops
Embodiment 2、25
[0084] Example 2. Preparation of 25% (volume fraction) human placental peptide stock solution + 0.1% (mass fraction) sodium hyaluronate eye drops
[0085] Human placental peptide stock solution 250ml
[0087] Sodium Hyaluronate 1g
[0088] 1mol / L sodium hydroxide appropriate amount
[0089] Add water for injection to 1000ml
[0090] Take 6.0g of sodium chloride and add appropriate amount of water for injection and dissolve it, stir well, adjust the pH to 7.2±0.2 with 1mol / L sodium hydroxide, then add 1g of sodium hyaluronate, soak to swell, stir and mix well. Autoclave for 30 minutes, cool, make up to 750ml of sterile water for injection, add 250ml of human placental peptide stock solution under aseptic conditions, mix well, sterile aliquot, 0.5ml each.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com