Process for continuously producing diethanolamine by using selective catalyst
A diethanolamine and catalyst technology, which is applied in the field of diethanolamine production technology, can solve the problems of high energy consumption and raw material consumption, poor product quality, long production cycle, etc., and achieve the effects of saving raw materials, reducing energy consumption, and reducing production processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
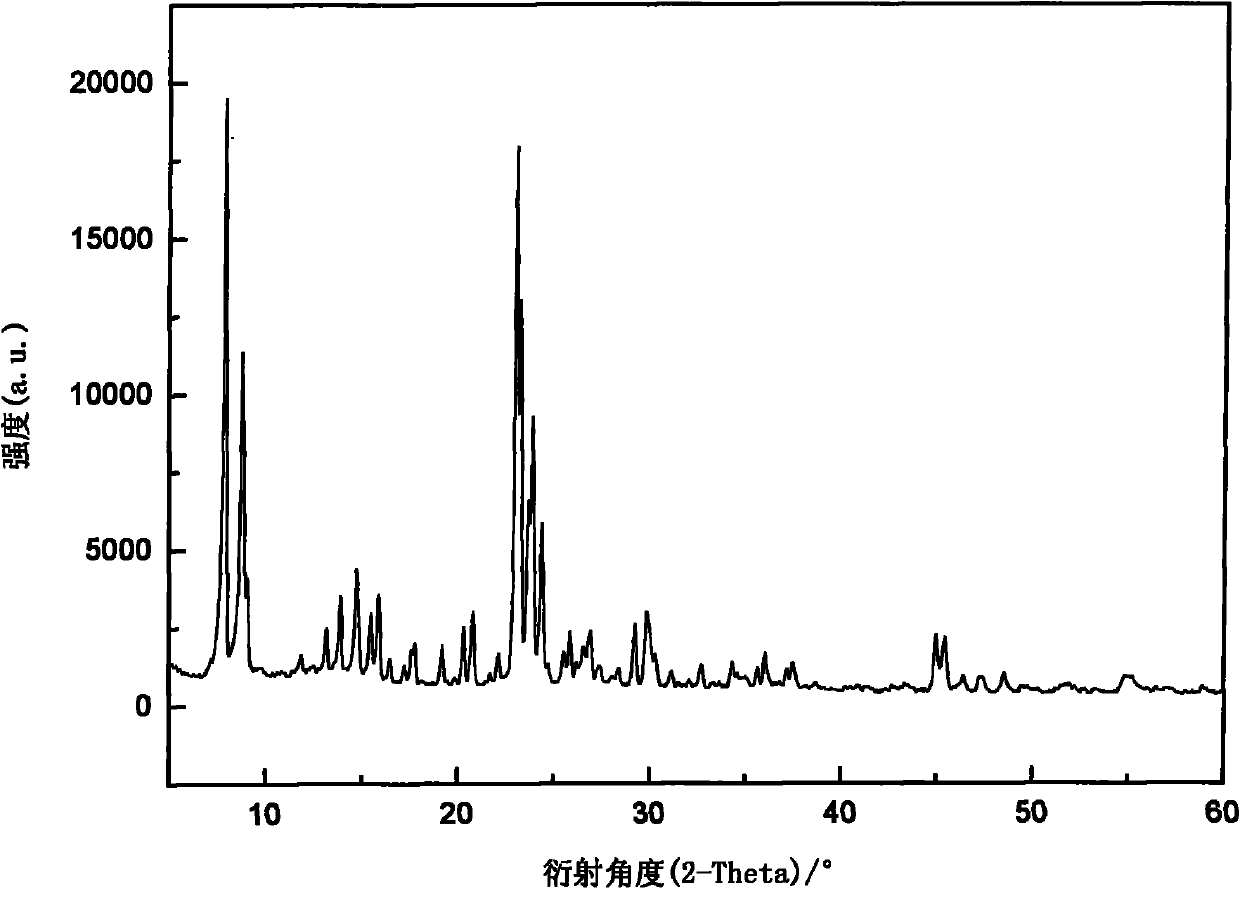
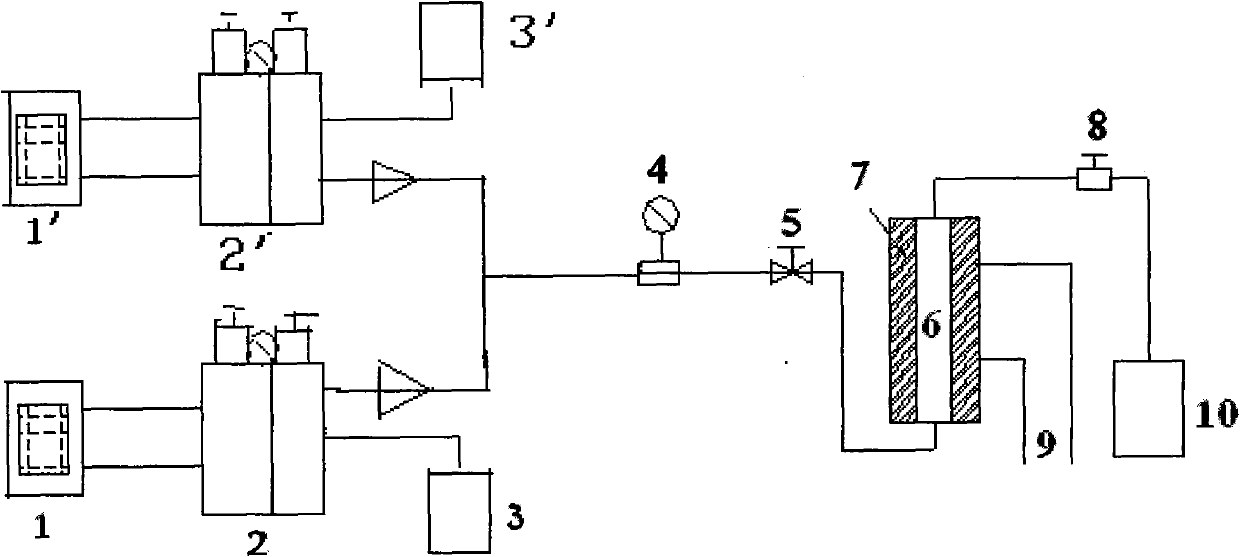
[0035] Select ZSM-5 zeolite molecular sieve, configure a supersaturated impregnating solution containing boric acid of 5% by weight of ZSM-5, after impregnating for 24 hours, suction filter, mother liquor for standby, after drying the filter cake for 12 hours, roasting at 550°C for 3 hours, and then use The remaining mother liquor was soaked, dried and roasted repeatedly, and the above steps were repeated 3 to 5 times to obtain B 2 o 3 / ZSM-5 catalyst, see figure 1 . Will B 2 o 3 / ZSM-5 catalyst is loaded into a fixed bed reactor after molding. Anhydrous liquid ammonia and ethylene oxide are fed into the fixed-bed reactor by two metering pumps at a ratio of 10:3, and the reaction pressure is controlled at 1.0-5.0MPa, the temperature is 40-160°C, and the volume space velocity is 1.0 ~3.0h -1 . The analysis result of the reactant after deamination treatment is: MEA:DEA:TEA=35:55:10.
Embodiment 2
[0037]Select ZSM-5 zeolite molecular sieve, configure a supersaturated impregnating solution containing zinc nitrate of 3% by weight of ZSM-5, after impregnating for 24 hours, suction filter, mother liquor for standby, after drying the filter cake for 12 hours, roasting at 550°C for 3 hours, and then Repeat impregnation, drying and roasting with the remaining mother liquor, and repeat the above steps 3 to 5 times to obtain a ZnO / H-ZSM-5 catalyst, which is shaped and loaded into a fixed-bed reactor. Anhydrous liquid ammonia and ethylene oxide are fed into the fixed-bed reactor by two metering pumps at a ratio of 10:3, and the reaction pressure is controlled at 1.0-5.0MPa, the temperature is 40-160°C, and the volume space velocity is 1.0 ~3.0h -1 . The analysis result of the reactant after deamination treatment is: MEA:DEA:TEA=26:58:16.
Embodiment 3
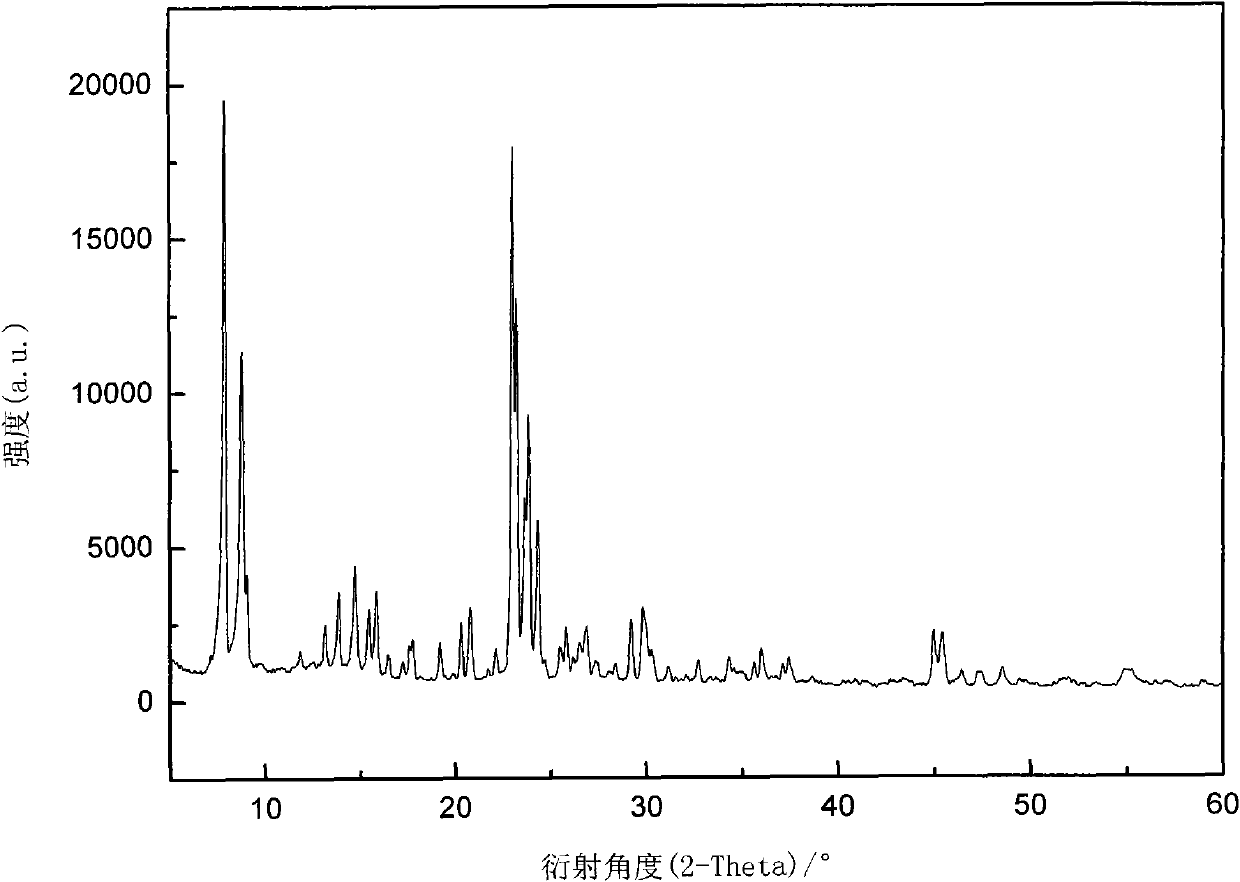
[0039] Select β zeolite molecular sieve, prepare a supersaturated impregnating solution containing boric acid of 8% by weight of β zeolite molecular sieve, after 24 hours of immersion, suction filter, mother liquor for standby, after drying the filter cake for 12 hours, roast at 550°C for 3 hours, and then use the remaining mother liquor Repeat dipping, drying, roasting, repeat the above steps 3 to 5 times to obtain B 2 o 3 / beta catalyst, see figure 2 . Will B 2 o 3 The / β catalyst is loaded into a fixed-bed reactor after being formed. Anhydrous liquid ammonia and ethylene oxide are fed into the fixed-bed reactor by two metering pumps at a ratio of 10:3, and the reaction pressure is controlled at 1.0-5.0MPa, the temperature is 40-160°C, and the volume space velocity is 1.0 ~3.0h -1 . The analysis result of the reactant after deamination treatment is: MEA:DEA:TEA=28:53:19.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com