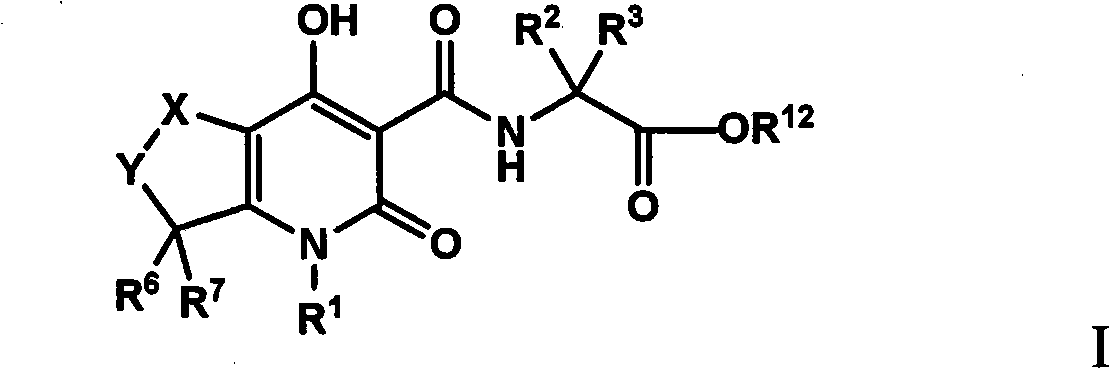
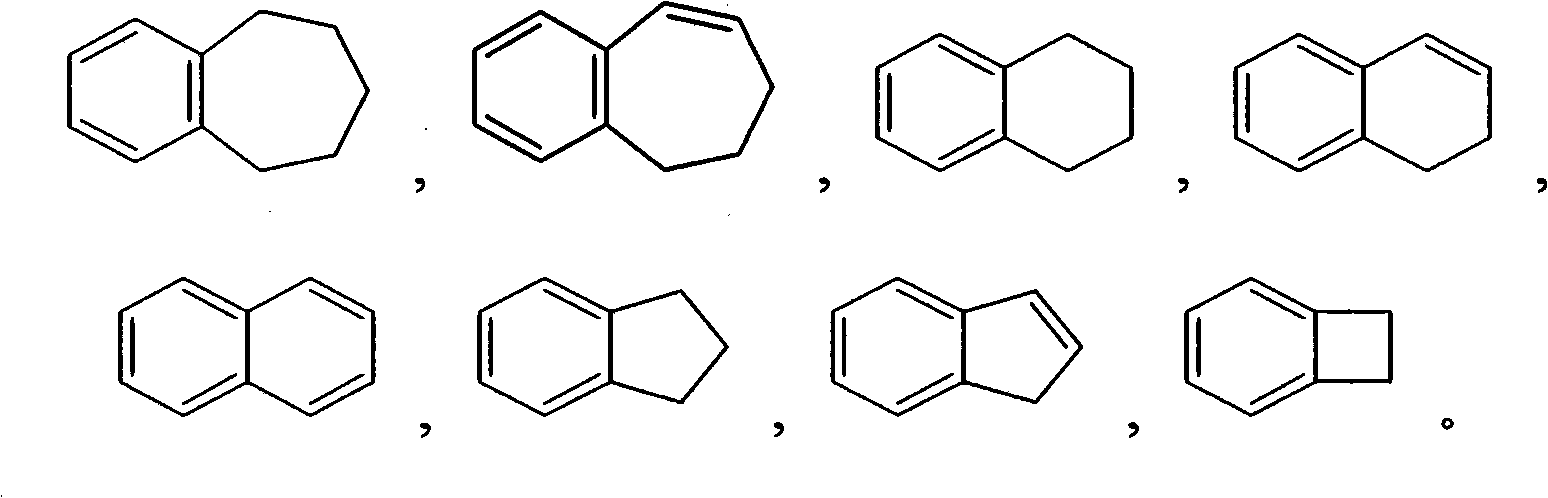
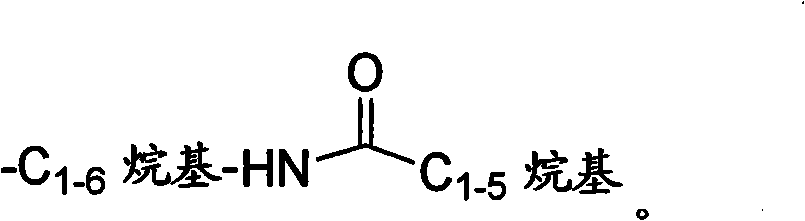
Tetrahydro-1H-pyrrolo fused pyridones
一种化合物、-CR4R5的技术,应用在药物组合、细胞外液疾病、杀生剂等方向,能够解决HIF-α羟基化反应效率低等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0175] Abbreviations used in describing the preparation of compounds of the invention:
[0176] AcOH acetic acid
[0177] Aq aqueous
[0178] Saline Saturated aqueous sodium chloride
[0179] CH 2 Cl 2 Dichloromethane
[0180] DMF N,N-Dimethylformamide
[0181] Dppf 1,1″-bis(diphenylphosphino)ferrocene
[0182] DBU 1,8-Diazabicyclo[5.4.0]undec-7-ene
[0183] DIEA N,N-Diisopropylethylamine
[0184] DMAP 4-N,N-Dimethylaminopyridine
[0185] DMF N,N-Dimethylformamide
[0186] DMSO Dimethyl Sulfoxide
[0187] EtOAc ethyl acetate
[0188] Et(et) ethyl
[0189] EtOH ethanol
[0191] G grams
[0192] h or hr hours
[0193] HCl hydrochloric acid
[0194] HPLC high performance liquid chromatography
[0195] IPA 2-propanol
[0196] i-PrOH Isopropanol
[0197] Mg mg
[0198] mL milliliter
[0199] Mmol millimole
[0200] MeCN acetonitrile
[0201] MeOH Methanol
[0202] Min minutes
[0203] ms or MS mass spectrometry
[0204]...
Embodiment 1
[0297]
[0298] N-({4-hydroxy-2-oxo-6-pent-4-enoyl-1-[4-(trifluoromethyl)benzyl]-2,5,6,7-tetrahydro-1H- Pyrrolo[3,4-b]pyridin-3-yl}carbonyl)glycine (E1-1)
[0299] At room temperature, to CH 2 Cl 2 To the product of intermediate 5 (112 mg, 0.204 mmol) in (0.7 mL) was added TFA (1.5 mL). The reaction was stirred for 25 min and concentrated. Use Et 2 O and hexane solidified the product. The solution was decanted and the solid was washed with hexane to yield the title compound E1-1. HPLC / MS: 494.0 (M+1); R t = 3.29 min.
[0300] Using a synthetic strategy similar to Intermediates 1 to 5 and Example 1, together with the appropriate amines in Step A of the Intermediate 4 process, Examples 2-4 as shown in Table 1 were prepared.
[0301] Table 1
[0302]
[0303]
Embodiment 5
[0305]
[0306] N-{[1-(4-bromobenzyl)-4-hydroxy-7-methyl-2-oxo-6-pent-4-enoyl-2,5,6,7-tetrahydro-1H- Pyrrolo[3,4-b]pyridin-3-yl]carbonyl}glycine (E5-1)
[0307] The title compound was prepared using a procedure analogous to Example 1, starting with Intermediate 2 and substituting 4-bromobenzylamine hydrochloride for 1-[4-(trifluoromethyl)phenyl] in Intermediate 4, Step A methylamine. HPLC / MS: 519.8 (M+1); R t = 3.11 min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com