Fluorene and spiro-fluorene substituted phenylpyridine iridium complex and preparation method and application thereof
A technology of phenylpyridine iridium and phenylpyridine, which is applied in the field of organic electroluminescent materials, can solve the problems of short phosphorescence lifetime and high quantum efficiency, and achieve the effects of low cost, easy synthesis, and reduced concentration quenching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
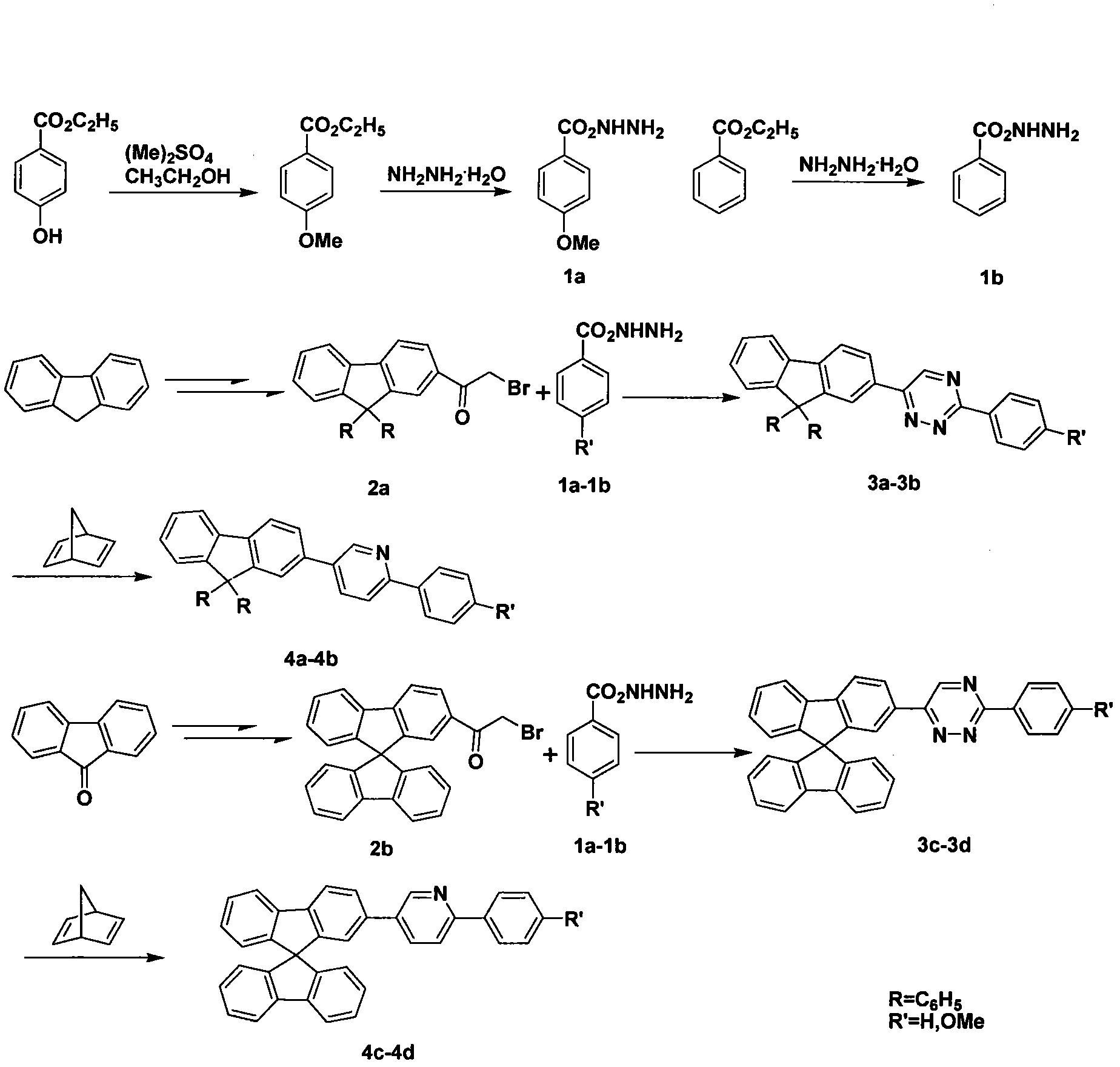
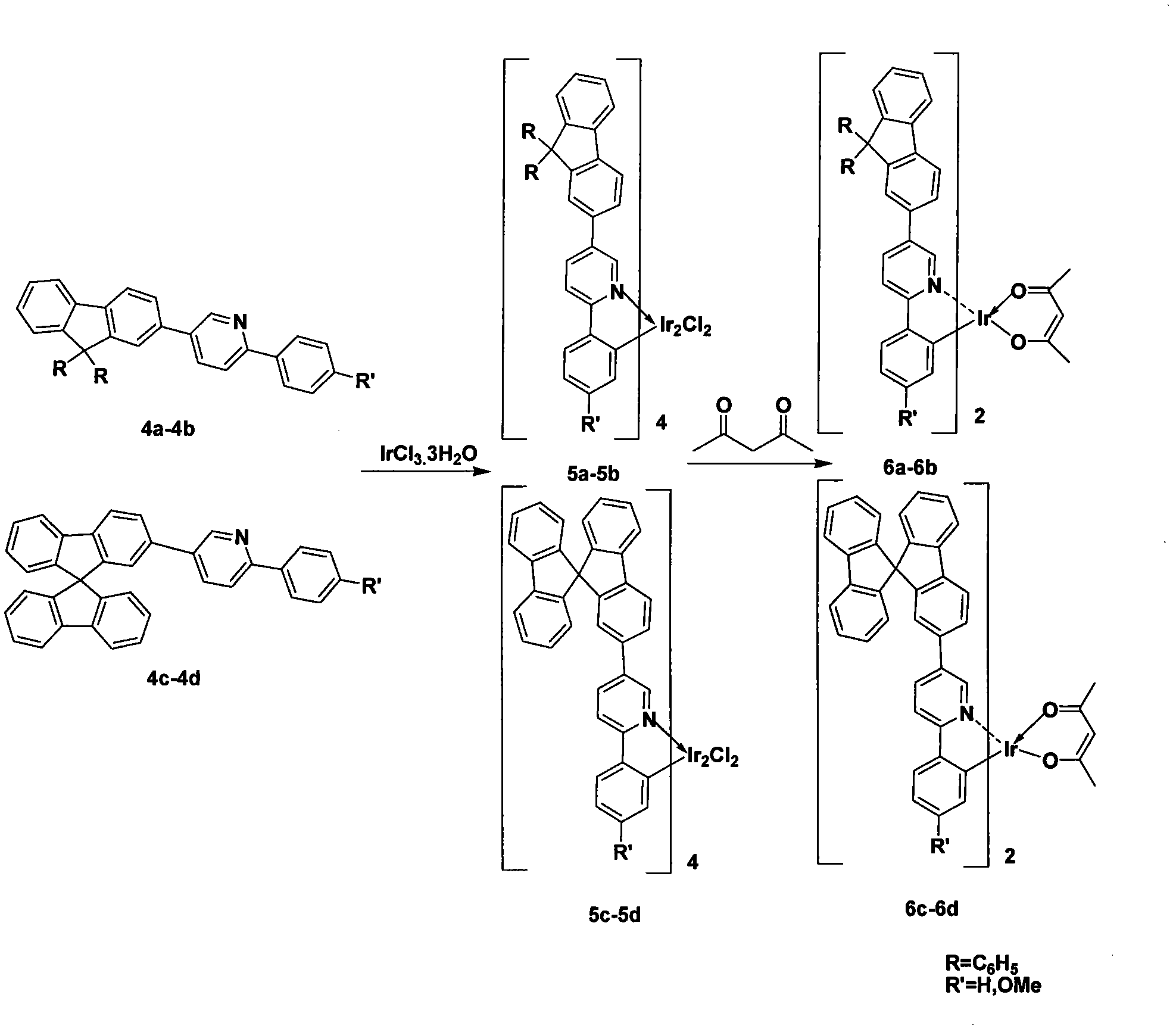
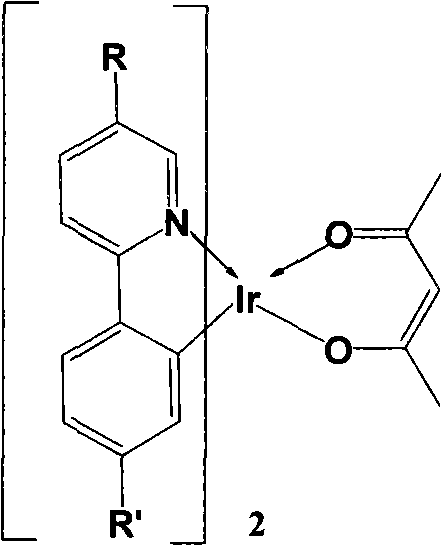
[0016] 1. Preparation of bis-[5-(9,9-dihexylfluorene)-2-phenylpyridine]iridium (acetylacetonate) complex 6b
[0017] (1) Preparation of 2-bromoacetyl-9,9'-dihexylfluorene 2a
[0018] Under ice bath, bromoacetyl bromide (2.07ml, 23.91mmol) was added dropwise to hexylfluorene (8g, 23.91mmol) and AlCl 3 (3.83g, 28.69mmol) of CH 2 Cl 2 solution, stirred for 20 min, then placed at room temperature and stirred for 1-2 hours. Pour into an ice bath of hydrochloric acid and stir, CH 2 Cl 2 Extracted, washed with water, saturated with Na 2 CO 3 Aqueous solution washing, water washing, anhydrous MgSO 4 After drying and concentration under reduced pressure, the residue was subjected to column chromatography (DCM:PE=1:4) to obtain a yellow liquid. 1 H NMR (CDCl 3 , 500MHz) δ: 8.02(d, J=6.6Hz, 2H), 7.81(m, 2H), 7.40(d, J=7.5Hz, 3H), 4.58(s, 2H), 2.07-2.02(m, 4H ), 1.14-1.04(m, 12H), 0.79-0.77(m, 6H), 0.64-0.61(m, 4H). 13 C NMR (CDCl 3 ,125MHz)δ:191.23,152.07,151.25,146.96,139.43...
Embodiment 2
[0028] Preparation of Bis-[5-(9,9')-dihexylfluorene-2-(4-methoxyphenyl)pyridine]iridium(acetylacetonate) 6a
[0029] (1) Preparation of 4-methoxybenzoic hydrazide 1a
[0030] Add ethyl p-methoxybenzoate (11.4g), 85% hydrazine hydrate (25ml), methanol (35ml) successively in a 250ml flask, heat to reflux for 24 hours, cool to precipitate a white solid, filter, and recrystallize from a small amount of ethanol. Melting point: 139-141°C. 1 HNMR (CDCl 3 , 500MHz) δ: 9.61(s, 1H), 7.81(d, J=8.8Hz, 2H), 6.98(d, J=8.7Hz, 2H), 4.41(s, 2H), 3.79(s, 3H).
[0031] (2) Preparation of 3-(4-methoxyphenyl)-6-(9,9'-dihexylfluorene)-1,2,4-triazine 3a
[0032] In a 100ml flask, add p-methoxybenzohydrazide (3.00g, 18.04mmol), 2-bromoacetyl-9,9'-dihexylfluorene (4.11g, 9.02mmol), sodium acetate (0.88g, 10.82mmol) ), ethanol (35ml), acetic acid (15ml). Add to reflux for 24 hours, cool, CH 2 Cl 2 Extraction, washing with water, saturated NaHCO 3 Aqueous wash, water wash, MgSO 4 After drying a...
Embodiment 3
[0040] Preparation of Bis-(5-(2-spirobifluorenyl)-2-phenylpyridine)iridium(acetylacetonate) 6c
[0041] (1) Preparation of 2-bromoacetylspirobifluorene 2b
[0042] In a 250ml three-necked flask, spirobifluorene (12g, 37.97mmol) was added to AlCl 3 (6.08g, 45.57mmol) in the dry dichloromethane solution, under ice-bath stirring, the dichloromethane (20ml) solution of bromoacetyl bromide (7.66g, 37.97mmol) is slowly added dropwise in the three-necked flask, After stirring at room temperature for 10 hours, pour into an ice bath of hydrochloric acid and stir, CH 2 Cl 2 Extracted, washed with water, saturated with Na 2 CO 3 Aqueous wash, water wash, MgSO 4 After drying and concentration under reduced pressure, the residue was subjected to column chromatography (DCM:PE=2:3) to obtain 11.35 g of a white solid, yield (68.35%). Melting point: 175-177°C. 1 H NMR (CDCl 3 , 500MHz) δ: 8.04(d, J=8.1Hz, 1H), 7.91(d, J=8.1Hz, 2H), 7.87(d, J=7.7Hz, 2H), 7.41-7.36(m, 3H), 7.35(s, 1H), ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com