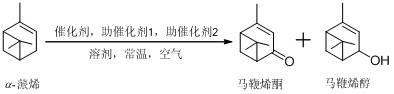
Preparation method of verbenol and verbenone through air oxidation of a-pinene at room temperature
A technology of verbenone and verbenol, applied in the field of preparation of verbenol and verbenone, can solve the problem that the yield of verbenone is less than 20%, the yield of verbenol is less than 12%, and the yield is less than 20%. % and other problems, to achieve the effect of short reaction time, cheap and easy-to-obtain catalyst, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 0.994g (7.3mmol) a- Add pinene to 10 mL of acetonitrile solvent, add 0.185 g (1.46 mmol) of catalyst ferric dichloride, add 0.263 g (1.46 mmol) of 1,10-phenanthroline, then add 22 mmol of hydrogen peroxide, and stir at 15 °C. The reaction was stopped after 2h. Centrifuge or filter to remove catalyst, dry with anhydrous sodium sulfate, and rotary evaporate to remove acetonitrile and hydrogen peroxide. With petroleum ether: a mixed solvent of ethyl acetate volume ratio of 5:1 was used as an eluent, and cocatalyst 1 (1,10-phenanthroline) and by-products were removed by column chromatography to obtain 0.078g verbenol (produced The rate is 7%), 0.941g verbenone (yield rate is 85.9%).
[0019] Verbenol: CDCl 3 , δ (ppm); NMR 1 H: 0.85 (3H,s,CH 3 , 8-H); 1.31 (3H, s, CH 3 , 10-H); 1.71 (t, J =1.59 Hz, CH 3 , 9-H); 2.00 (m, 1H, 4-H); 2.17 (m, 1H,5-H); 2.26 (m, 1H, 6-H); 4.43 (m, 1H, 1-H); 5.32 (m,1H, 2-H).
[0020] Verbolenone: CDCl 3 , δ (ppm); NMR 1 H: 0.98 (3H,...
Embodiment 2
[0022] 0.994g (7.3mmol) a- Add pinene to 10 mL of dichloromethane solvent, add 0.277g (1.46mmol) tin dichloride, add 0.263g (1.46mmol) 1,10-phenanthroline, then add 29.2mmol peracetic acid, at 25°C After stirring, stop the reaction after 3h. Remove the catalyst by centrifugation or filtration, dry with anhydrous sodium sulfate, and rotary evaporate to remove dichloromethane and peracetic acid. With sherwood oil: the mixed solvent that ethyl acetate volume ratio is 5: 1 is eluent, removes cocatalyst 1 (1,10-phenanthroline) and by-product through column chromatography, obtains 0.973g verbenol (product The yield was 87.6%), 0.035g verbenone (the yield was 3.2%).
Embodiment 3
[0024] 0.994g (7.3mmol) a- Add pinene to 10mL acetic acid solvent, add 0.098 g (0.73mmol) copper dichloride, add 0.114g (0.73mmol) 2,2'-bipyridine, then add 14.6mmol tert-butyl hydroperoxide, at 40°C After stirring, stop the reaction after 1h. The catalyst was removed by centrifugation and filtration, dried over anhydrous sodium sulfate, and rotary evaporated to remove acetic acid and tert-butyl hydroperoxide. With sherwood oil: the mixed solvent that ethyl acetate volume ratio is 5: 1 is eluent, removes 2,2'-dipyridine and by-product through column chromatography, separates and obtains 0.087g verbenol (yield rate 7.8% ), 0.902g verbenone (82.3% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com