Benzothiadiazole-based multi-arm conjugated molecules as well as preparation method and application thereof
A technology of benzothiadiazole and conjugated molecules, which is applied in the field of multi-arm conjugated molecules based on bibenzothiadiazole and its preparation and application, can solve the problem of affecting the service life of devices, increasing production costs, and changing luminous efficiency. Low-level problems, to achieve the effect of good thermal stability, good processability and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The synthetic route of small molecule 1 is as follows.
[0063]
[0064] Add bibenzothiadiazole (540mg, 2mmol) and 8ml hydrobromic acid to a 50ml single-necked bottle, slowly add liquid bromine (1.92g, 12mmol, dissolved in 6ml hydrobromic acid) at room temperature, after the addition, 110 °C reflux for one day. After cooling to room temperature, the suspension was washed with saturated sodium bisulfite solution to remove excess bromine, filtered, the solid was washed with a large amount of water, dried and purified by silica gel column chromatography to obtain light yellow solid 1a (279 mg, 40%). 1 H NMR (400MHz, CDCl 3 ): δ8.13(s, 1H), 8.11(d, J=8.9Hz, 1H), 8.06(d, J=8.9Hz, 1H), 7.78(d, J=8.9Hz, 1H), 7.70(d , J=8.9Hz, 1H). EI-MS: 347.9141; Calculated value: 347.9139. Elemental analysis calculated value (C 12 h 5 N 4 S 2Br): C, 41.27; H, 1.44; N, 16.04. Found: C, 41.12; H, 1.82; N, 16.00%.
[0065] (2)1b
[0066] Add 1a (942mg, 2.7mmol), 3-dodecyl-5-tributylt...
Embodiment 2
[0073] The synthetic route of small molecule 2 is as follows
[0074]
[0075] Add 2-bromo-3-dodecylthiophene (993mg, 3mmol), triphenylamine monotin (2.4g, 4.5mmol) and 20ml toluene solution in the 50ml two-necked bottle, after purging the air in the reaction vessel with nitrogen, add Tetrakis(triphenylphosphine)palladium (115mg, 0.1mmol), reflux at a temperature of 100-120°C for 48 hours, cool to room temperature, add excess potassium fluoride solution relative to the molar amount of triphenylamine monotin in the container and stir for 2 Hour. The organic phase was extracted with chloroform, washed twice with water, dried over anhydrous magnesium sulfate, spin-dried to dry the solvent, and purified by silica gel column chromatography to obtain light yellow oily liquid 2a (1.29 g, 87%).
[0076] EI-MS: 495.2964, Calculated: 495.2960.
[0077] (2)2b
[0078] Add 2a (495mg, 1mmol) and 10ml dry tetrahydrofuran solution into a 50ml three-necked flask, cool to -78°C, vent the...
Embodiment 3
[0085] The synthetic route of small molecule 3 is as follows
[0086]
[0087] Add bibenzothiazole (540mg, 2mmol) and 8ml of hydrobromic acid into a 50ml single-necked bottle, slowly add liquid bromine (1.92g, 12mmol, dissolved in 6ml of hydrobromic acid) at room temperature, after the addition, reflux at 110°C One day, liquid bromine (1.92 g, 12 mmol, dissolved in 6 ml of hydrobromic acid) was then added, and the reaction was continued for one day. After cooling to room temperature, the suspension was washed with saturated sodium bisulfite solution to remove excess bromine, filtered, the solid was washed with plenty of water, dried and purified by silica gel column chromatography to give 3a (355 mg, 35%) as a pale yellow solid. 1 H NMR (400MHz, CDCl 3 ): δ 8.10 (d, J=9.0 Hz, 1H), 7.84 (s, 1H), 7.57 (d, J=9.0 Hz, 1H). EI-MS: 507.7310, Calculated: 507.7308. Elemental analysis calculated value (C 12 h 3 N 4 S 2 Br 3 ): C, 28.43; H, 0.60; N, 11.05. Found: C, 28.19; H, 0...
PUM
| Property | Measurement | Unit |
|---|---|---|
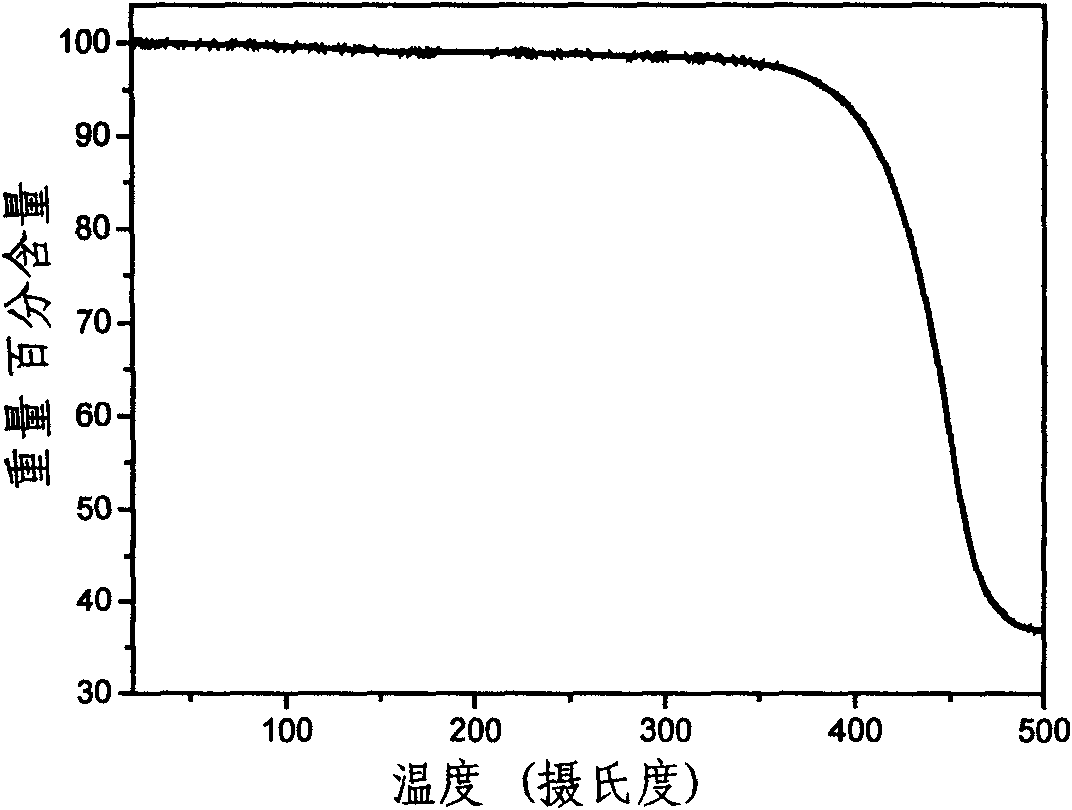
| thermal decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com