Ractopamine residual time resolution immunoassay kit and detection method thereof
A technology of ractopamine and detection kits, which is applied in the field of immunoassays, and can solve problems such as lack of ractopamine time-resolved immunoassay detection kits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] The preparation of embodiment 1 hapten
[0081] The synthetic route to the ractopamine hapten is shown below:
[0082]
[0083] Dissolve ractopamine hydrochloride and succinic anhydride in anhydrous pyridine and stir at room temperature. After the reaction was completed, pyridine and unreacted raw materials were removed by extraction, and evaporated to dryness on a vacuum rotary evaporator under reduced pressure to obtain the crude product of the hapten ractopamine-succinate. The above post-reaction treatment process was monitored in real time by TLC. The crude product was purified by column chromatography to obtain a relatively pure hapten, which was identified by ESI-MS.
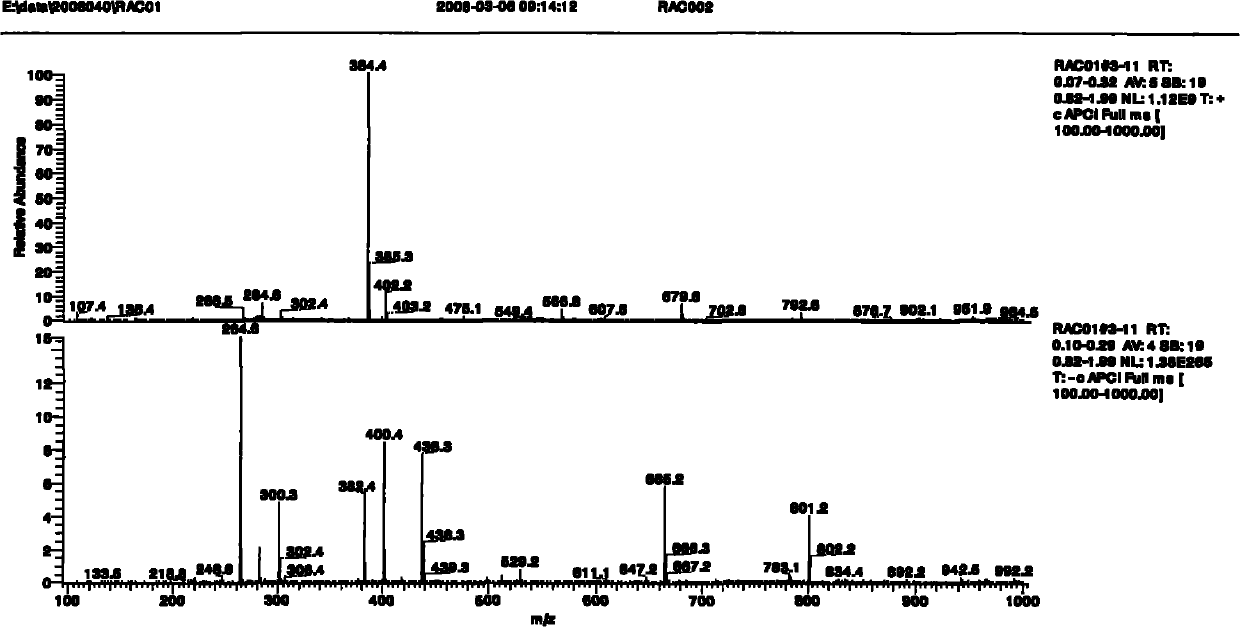
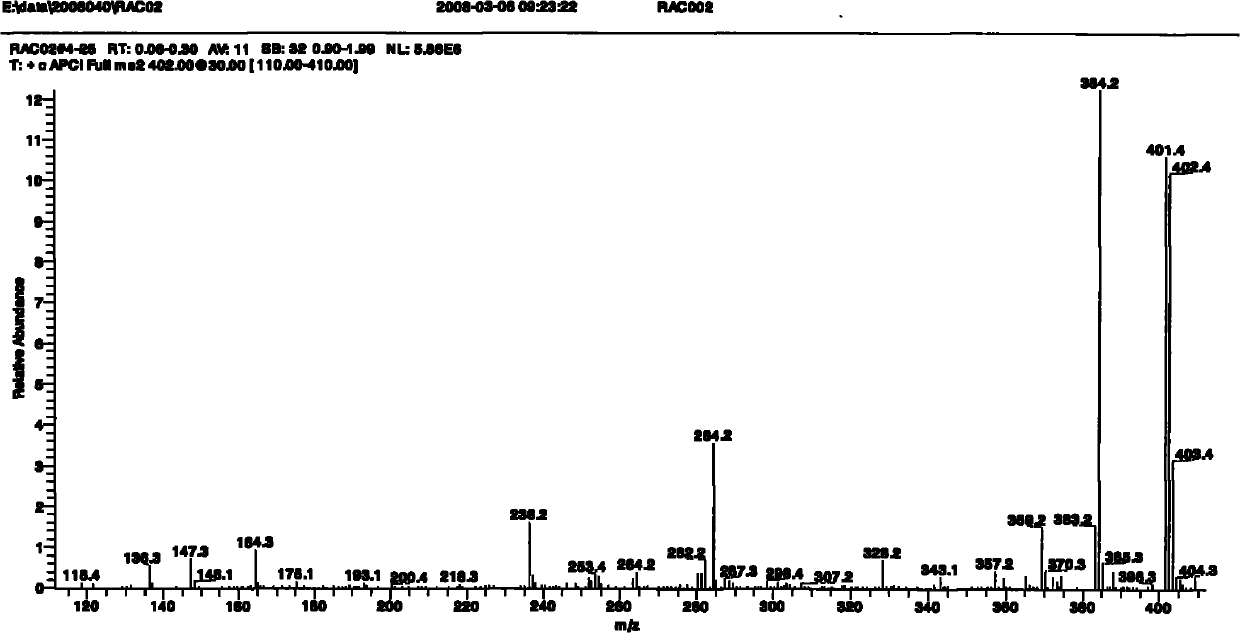
[0084] (+)ESI-MS full scan mass spectrum of ractopamine hapten see figure 1 As shown, the molecular ion peak of m / z 400 appears in the figure, which corresponds to the molecular mass of the hapten. In order to further confirm its structure, (+) ESI-MS2 analysis was carried out on the molecula...
Embodiment 2
[0085] The preparation of embodiment 2 antigen
[0086] Preparation of ractopamine antigen:
[0087] a. Dissolve 50 μmol / L ractopamine hapten in 1 mL of dimethylformamide (DMF), then add equimolar dicyclohexylcarbodiimide (DCC) and N-hydroxysuccinimide to the solution Amine (NHS), let it react overnight at room temperature;
[0088] b. Centrifuge, take 800 μL of supernatant, slowly add to 4 mL of 15 mg / mL BSA or OVA carrier protein carbonic acid buffer solution, and then react for 4 hours under magnetic stirring;
[0089] c. After the reaction is completed, put it into a dialysis bag, first dialyze twice with distilled water, and then dialyze with 0.8% normal saline to obtain the product;
[0090] d. The binding ratio was determined by ultraviolet scanning (Chen Xinxin et al., 1998), and finally the antigen was concentrated or lyophilized to obtain the ractopamine immunogen and the coating agent, which were stored in a refrigerator at -20°C.
Embodiment 3
[0091] The preparation of embodiment 3 antibody
[0092] Preparation of monoclonal antibodies:
[0093] Animal immunization procedure: Balb / c mice were used as immunized animals, the ractopamine hapten and bovine serum albumin conjugate was used as the immunogen, the immunization dose was 60 μg / mouse, and the immunogen was mixed with the same amount of Freund’s The complete adjuvant was mixed to make an emulsifier, which was injected intraperitoneally. The same dose of immunogen and the same amount of Freund's incomplete adjuvant were mixed and emulsified at intervals of 3 weeks, and a booster immunization was given.
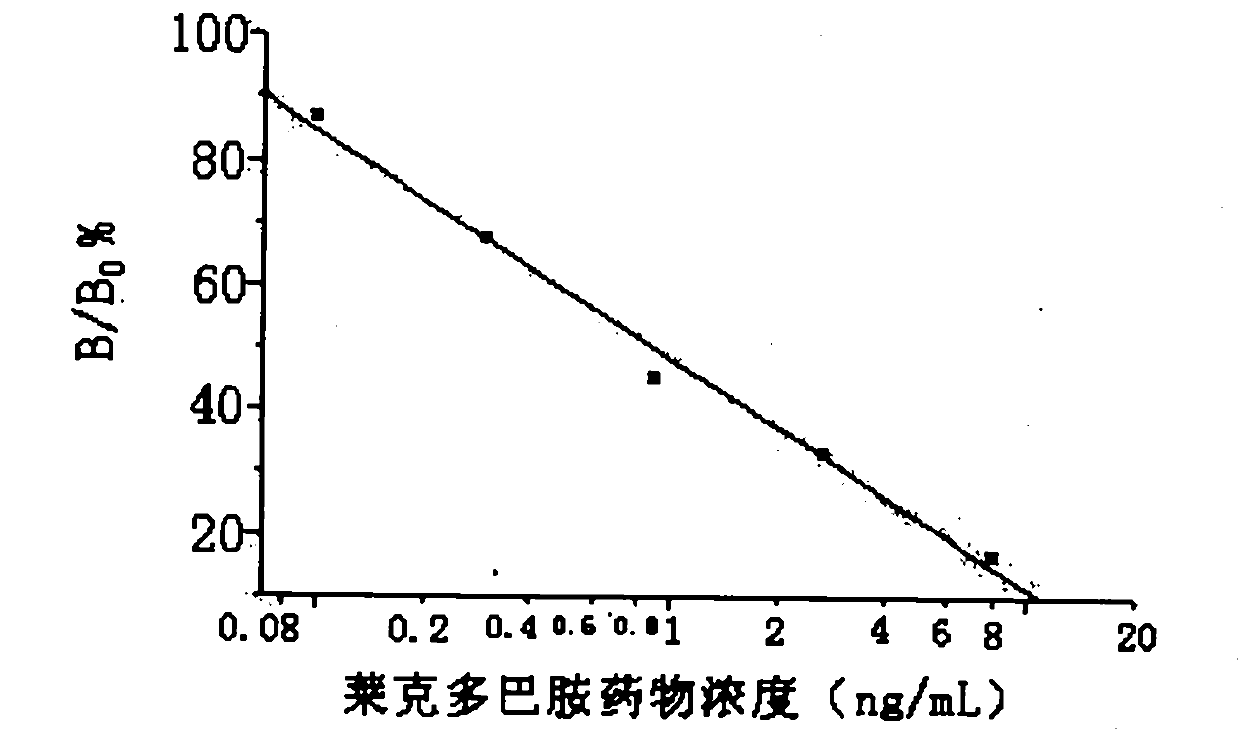
[0094] Cell fusion and cloning: Splenocytes from immunized Balb / c mice were fused with SP2 / 0 myeloma cells at a ratio of 4:1. Cell supernatants were measured by indirect competitive time-resolved immunoassay, and positive wells were screened. The positive wells were cloned by microcloning until a hybridoma cell line stably secreting the monoclonal antibody was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com