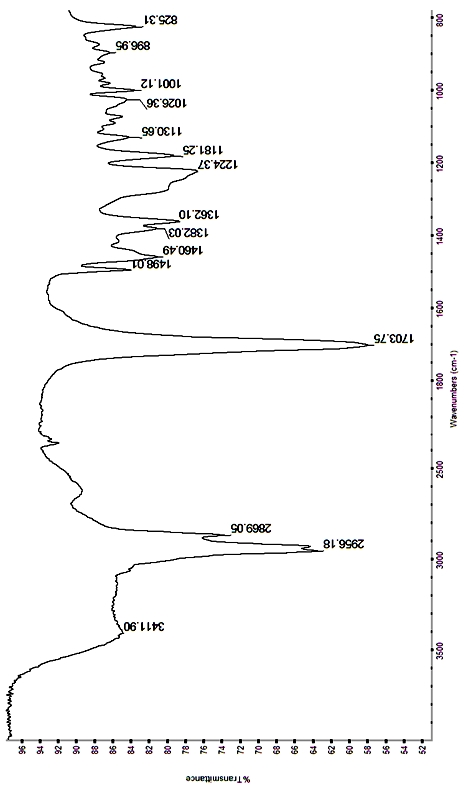
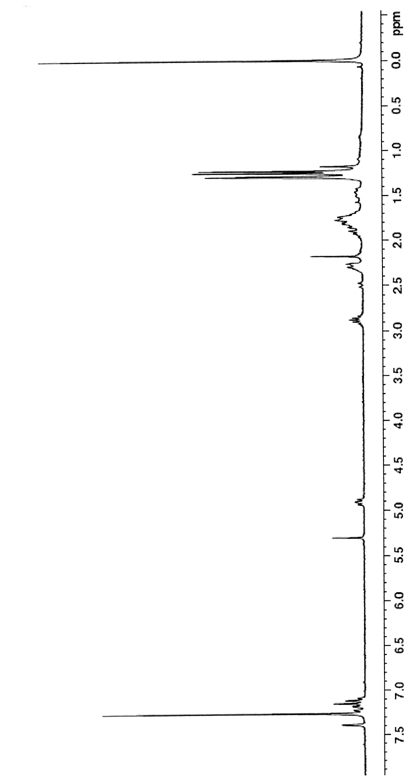
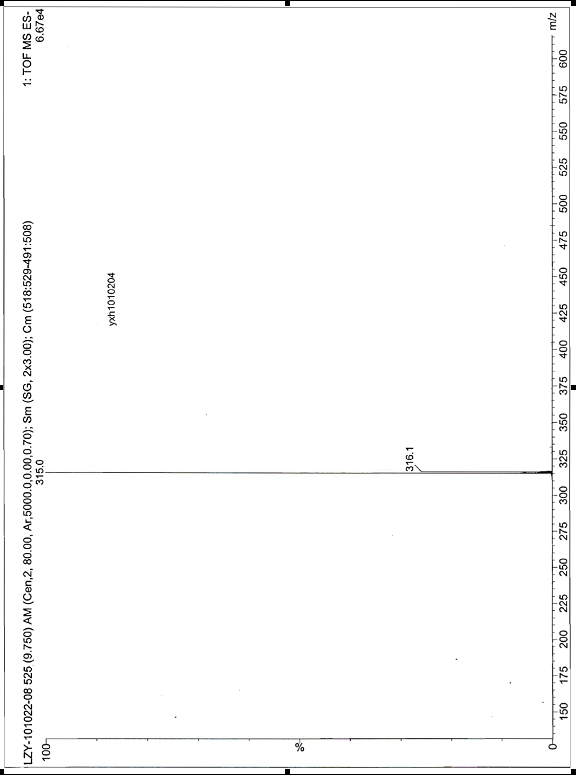
Method for preparing 7-hydroxydehydroabietic acid
A technology of dehydroabietic acid and hydroxyl group, applied in the field of preparation of pharmaceutical intermediate compounds, can solve the problems of low reaction selectivity, cumbersome reaction steps, complicated oxidation products, etc., and achieves cost reduction, good reproducibility, and reduced reaction effect of steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A kind of preparation method step of 7-hydroxydehydroabietic acid is:
[0030] Step 1: Add 2 parts by mass of rosin to 5 to 100 parts by mass of an organic solvent with 2 to 6 carbons, stir evenly, and heat to completely dissolve the rosin; the organic solvent with 2 to 6 carbons used is dichloromethane, 1, Any one or more of 4-dioxane, tetrahydrofuran or acetone.
[0031] Step 2: Add the rosin solution dissolved in the previous step into a fixed-bed reactor equipped with an oxidant. The fixed-bed reactor is kept at a temperature of 30-70°C, and the reaction time is ≤60 min. A solution of 7-hydroxydehydroabietic acid is obtained; the oxidizing agent is any one of dichlorodicyanoquinone, selenium dioxide and manganese dioxide.
[0032] Step 3: Filter the 7-hydroxydehydroabietic acid solution in the previous step, distill, recrystallize, and dry in vacuum to obtain pure 7-hydroxydehydroabietic acid. For recrystallization, any one of acetone, ethanol, and acetic acid is ...
Embodiment 2
[0034] Put 2 parts of rosin into 10 parts of dichloromethane solvent, heat to 50°C, and dissolve them all under stirring. The dissolved abietic acid is added to the fixed-bed reactor equipped with dichlorodicyanoquinone. The reaction was heated for 5 min to obtain a 7-hydroxydehydroabietic acid solution; the content of 7-hydroxydehydroabietic acid in the solution was analyzed by gas chromatography, and its content was 76%. The reaction solution was filtered, distilled, and recrystallized from acetone to obtain the target product, and the content of 7-hydroxydehydroabietic acid was more than 95% according to liquid chromatography (HPLC).
Embodiment 3
[0036] Put 2 parts of rosin into 10 parts of 1,4-dioxane solvent, heat to 50°C, and dissolve them all under stirring. Add the dissolved rosin to the MnO 2 in a fixed bed reactor. The reaction was heated for 20 min to obtain a 7-hydroxydehydroabietic acid solution; the content of 7-hydroxydehydroabietic acid in the solution was analyzed by gas chromatography, and its content was 78%. The reaction solution was filtered, distilled, and recrystallized from acetic acid to obtain the target product, and the content of 7-hydroxydehydroabietic acid was more than 96% according to liquid chromatography (HPLC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com