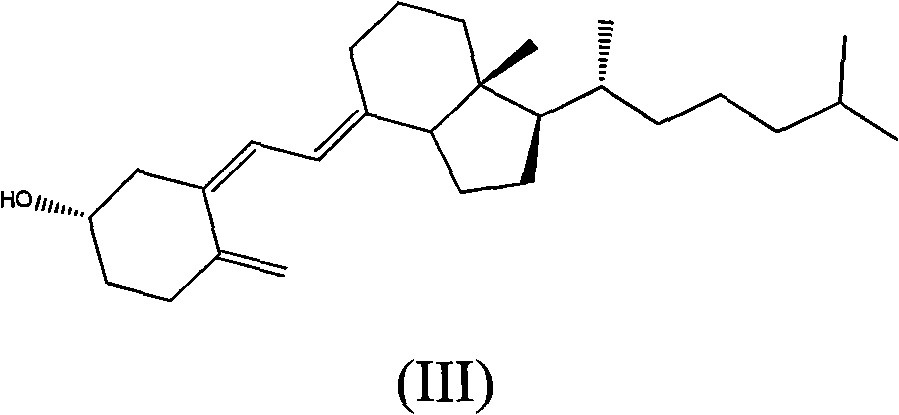
Method for preparing 7beta-hydroxyl-3beta cholesterol acetate from hydroxylase 3beta-cholesterol acetate
A technology of cholesterol acetate and β-cholesterol, which is applied in the field of enzymatic hydroxylation of 3β-cholesterol acetate to prepare 7β-hydroxy-3β-cholesterol acetate, which can solve problems such as industrial production difficulties, environmental pollution, and complex synthesis operations , to achieve good technical application and industrialization prospects, reduce production costs, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A method for enzyme hydroxylation of 3β-cholesterol acetate to prepare 7β-hydroxyl-3β-cholesterol acetate, the specific steps of the method are as follows:
[0044]10 mg of crude enzyme of cytochrome P450 hydroxylase (cytochrome P450, Cyp27b1, locus tag: NP_034139 from gene bank) was dissolved in 10 mL (2 mM, pH=8.9) of PBS buffer solution.
[0045] Take out 10mL and add in the Erlenmeyer flask of 100mL, add 20mL PBS phosphate buffer solution (2mM, pH=8.9), continue to add 5mL concentration and be the 3β-cholesterol acetate solution (pH=8.9, 30% ethanol) of 10mM, 8 mL of hydrogen peroxide solution (pH=8.9) with a concentration of 10 mM was fully stirred in a shaker, the stirring speed was 200 r / min, the temperature was fixed at 37 ° C, and the reaction was performed for 2 hours. The mixed reagent of ether is used as a developing agent, and the end point of the reaction can be judged by monitoring the concentration of newly generated spots and the concentrations of remai...
Embodiment 2
[0049] A method for enzyme hydroxylation of 3β-cholesterol acetate to prepare 7β-hydroxyl-3β-cholesterol acetate, the specific steps of the method are as follows:
[0050] 20 mg of crude enzyme of cytochrome P450 monooxygenase (CYP98A3, locus tag: AT2G40890 from gene bank) was dissolved in 10 mL (5 mM, pH=8.0) of PBS buffer solution.
[0051] Take out 10mL and add it to a 100mL Erlenmeyer flask, add 20mL of PBS phosphate buffer solution (5mM, pH=8.0), and continue to add 5mL of 10mM 3β-cholesterol acetate solution (pH=8.0, 20% ethanol ), 8 mL of hydrogen peroxide solution (pH=8.0) with a concentration of 10 mM, fully stirred and reacted in a dynamic stirring device, the stirring speed was 200 r / min, and the temperature was fixed at 35 ° C for 0.5 hours. The formation of compound 7β-hydroxy-3β-cholesterol acetate and the disappearance of compound 3β-cholesterol acetate in phase chromatography were used to determine the end point of the reaction.
[0052] After the reaction, th...
Embodiment 3
[0055] A method for enzyme hydroxylation of 3β-cholesterol acetate to prepare 7β-hydroxyl-3β-cholesterol acetate, the specific steps of the method are as follows:
[0056] 15 mg of crude enzyme of Caenorhabditis elegans monooxygenase (Caenorhabditis elegans monooxygenase, locus tag: T19B4.1 from gene bank) was dissolved in 10 mL (5 mM, pH=7.0) of PBS buffer solution.
[0057] Take out 10mL and add in the Erlenmeyer flask of 100mL, add 20mL PBS phosphate buffered solution (5mM, pH=7.0), continue to add 3β-cholesterol acetate solution (pH=7.0, 25% acetone) that 5mL concentration is 10mM, 8 mL of hydrogen peroxide solution (pH=7.0) with a concentration of 10 mM was fully stirred in a shaker to react at a stirring speed of 130 r / min, and the temperature was fixed at 35°C for 1.5 hours. During the reaction, TLC silica gel plate was used to monitor, using ethyl acetate and petroleum ether The mixed reagent is used as a developing agent, and the end point of the reaction is judged by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com