Double-layer moxifloxacin suppository and preparation method thereof
A moxifloxacin hydrochloride, double-layer technology, applied in the field of double-layer suppositories, can solve the problems of not showing the superiority of suppositories, and achieve the effects of avoiding toxic and side effects, good curative effect and long duration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
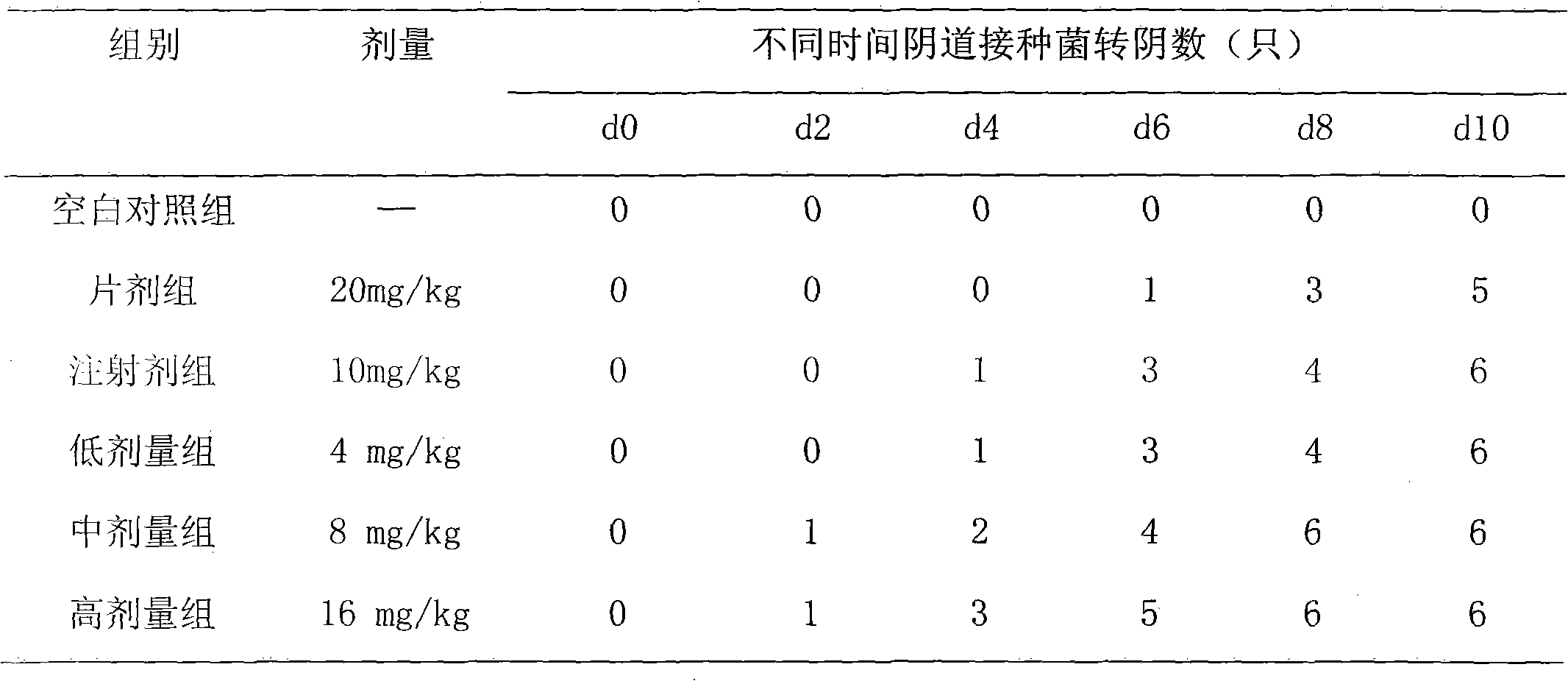
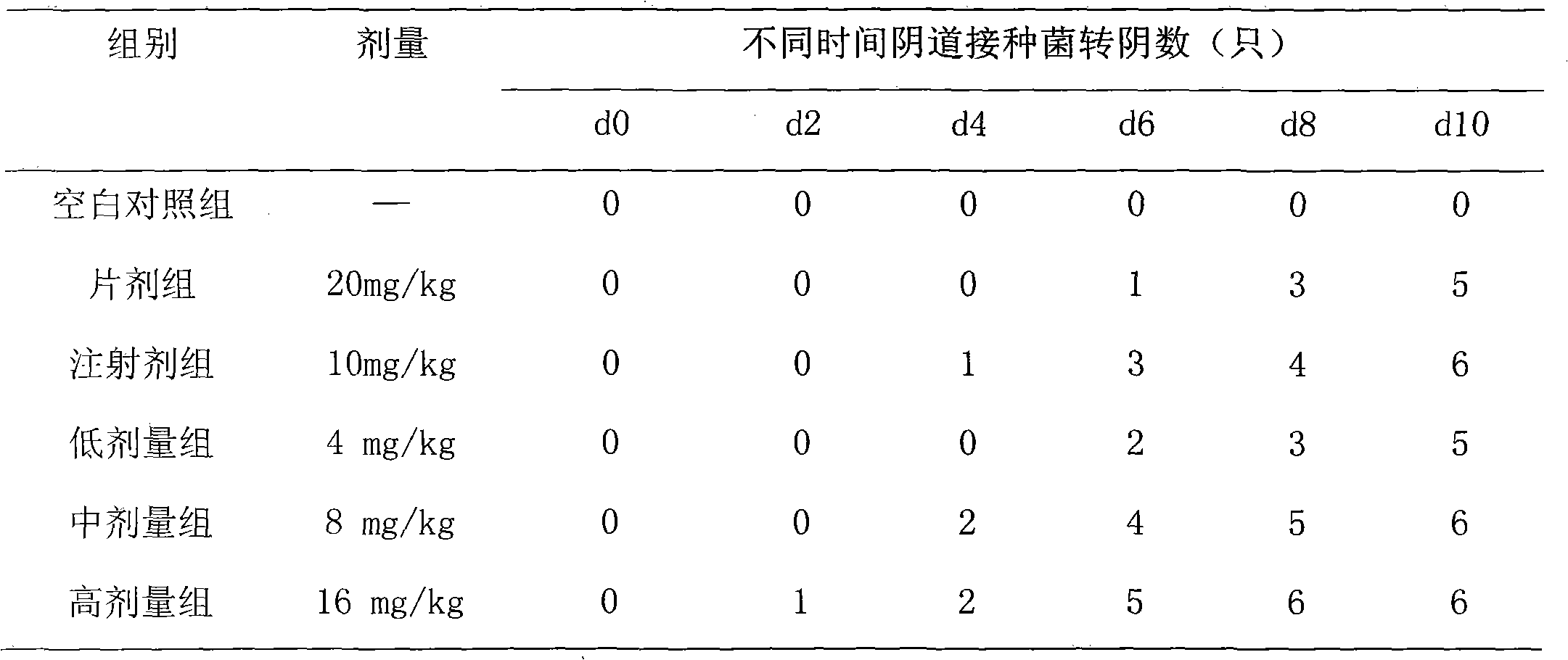
[0021] Preparation of moxifloxacin double-layer suppository: preparation of the slow-release part Take the water-soluble matrix of 135g of gelatin and 75g of glycerin, heat and melt in a water bath, add 12g of moxifloxacin hydrochloride under stirring, mix well, and pour it into the vaginal suppository while heating In the mold, cool, demould, and set aside; preparation of the quick-release part Take an appropriate amount of PEG 1000156g, PEG 40008g, water 18ml, glycerin 10g, heat and melt in a water bath, add 8g of moxifloxacin hydrochloride under stirring, mix well and multiply Pour the heat into the vaginal suppository mold, and after cooling down slightly, insert the prepared sustained-release suppository to the end (try to insert from the center), and it is ready. Each suppository contains 200 mg of moxifloxacin hydrochloride.
Embodiment 2
[0023] In Vitro Dissolution Determination: Comparison of In Vitro Dissolution of Double-layer Suppositories and Common Suppositories (1) In Vitro Dissolution Determination of Double-layer Suppositories: Using the Rotating Basket Method, Take a Moxifloxacin Double-Layer Suppository in Example 1 and Place It in Stainless Steel In the mesh basket, the outer surface is covered with a 0.8um microporous filter membrane to form a cylinder, and then the cylinder is placed in the rotating basket. The dissolution medium is 900ml of artificial intestinal fluid, the temperature is (37.5±0.1)°C, and the rotation speed is 150r / min. Regularly sample 5ml, filter (replenish medium 5ml immediately after each sampling), discard the primary filtrate, accurately measure 4ml of the subsequent filtrate, put it in a 10ml volumetric flask, add artificial intestinal juice to the scale, and measure the absorption at a wavelength of 296nm with the dissolution medium as a blank Degree A, calculate the medi...
Embodiment 3
[0025] Stimulation test: 6 healthy adult rabbits were taken, weighing (3.0±0.5) kg, and the rabbits were divided into two groups, namely the drug treatment group and the blank control group. The moxifloxacin double-layer suppository in Example 1 and the excipient with the same matrix but without moxifloxacin were placed into the rectum of the rabbit, administered once every other day for 8 days, and killed 48 hours after the last administration , Take out the local mucous membrane, observe that there is no hyperemia and redness in the medication group, and the observation results are no different from those in the blank control group.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com