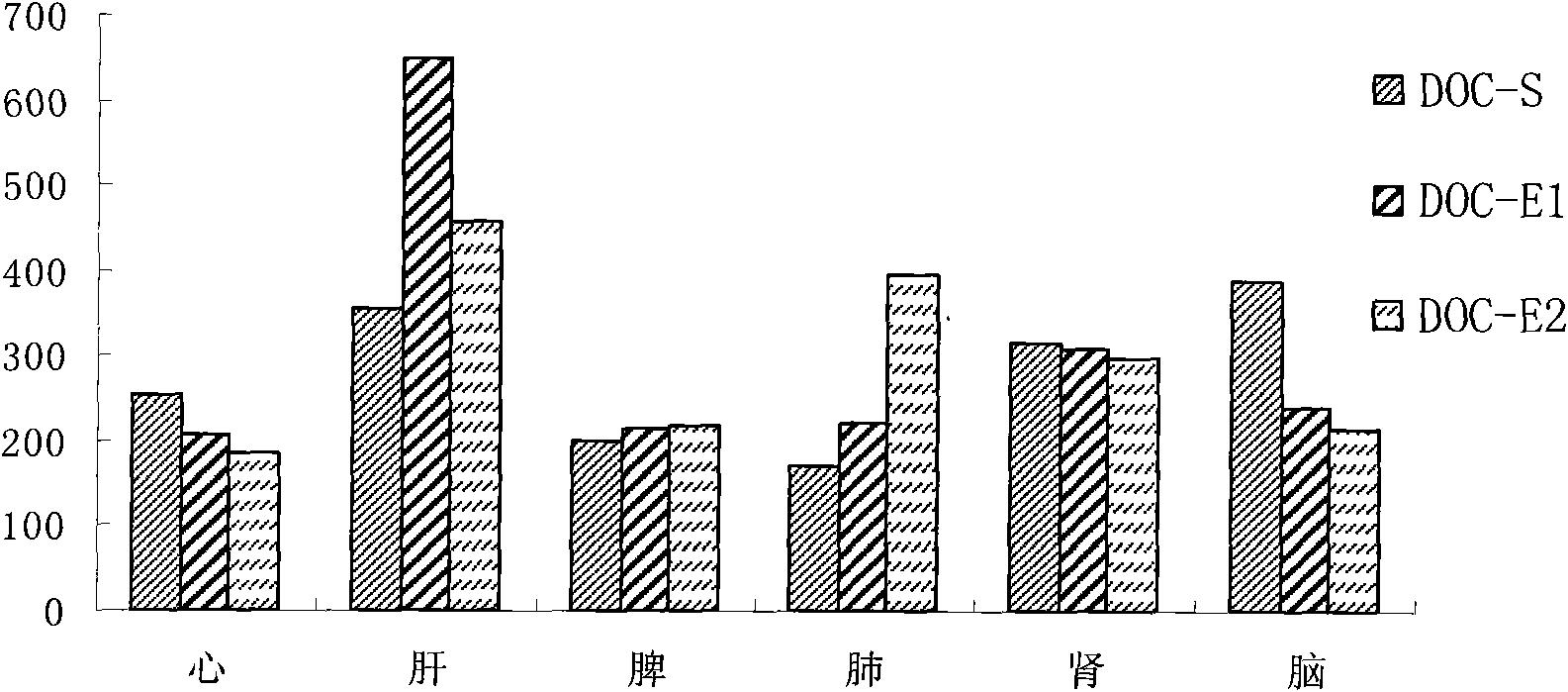
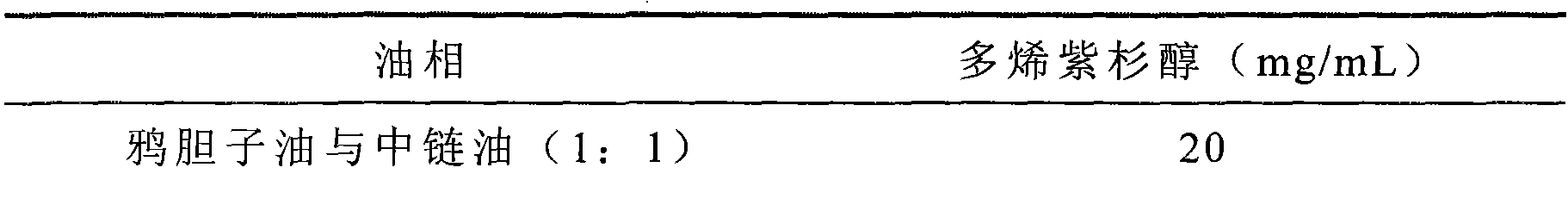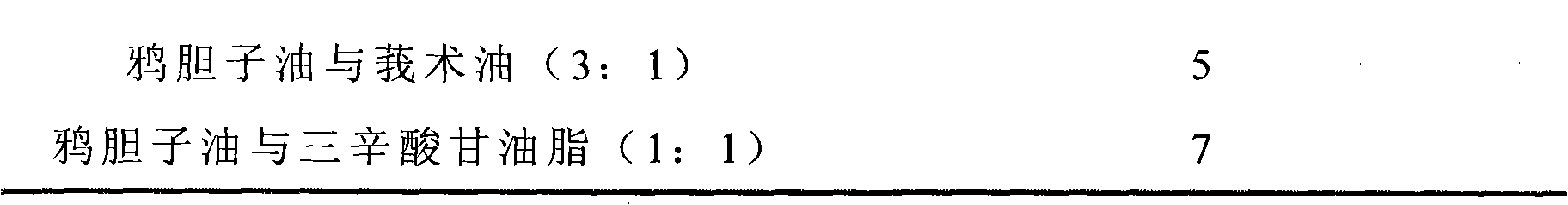Compound docetaxel ester microsphere injection and preparation method thereof
A technology of compound docetaxel and injection, which is applied in the field of compound docetaxel lipid microsphere injection and preparation, can solve the problems such as the decrease of drug content, and achieve the effects of improving solubility, improving drug distribution, and increasing sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The compound docetaxel lipid microsphere injection of the present invention is prepared by the following raw materials by weight and number ratio, docetaxel: Brucea javanica oil: medium chain oil: egg yolk lecithin: polyethylene glycol-12-hydroxyl Stearic acid ester: glycerin: sterile water for injection = 0.5: 30: 30: 10: 10: 20: 700.
[0032] 10g refined egg yolk lecithin (Germany Lipoid company) and polyethylene glycol-12-hydroxystearate of 10g are dispersed in the composite oil phase that 30g injection grade Brucea javanica oil and 30g medium chain oil form, by the above weight Precisely weigh 0.5g of docetaxel and add it to the above oil phase to dissolve, add 700g of sterilized water for injection at about 55-65°C and 20g of glycerin, homogenize at 20000rpm for 5 minutes to form colostrum, add colostrum In the high-pressure homogenizer (Niro-Soavi, Italy), pass N 2 Protection, the initial pressure is 20-30MPa, cycle for 2 minutes, then rise to 80-100Mpa, cycle 6 ...
Embodiment 2
[0034] The compound docetaxel lipid microsphere injection of the present invention is prepared by the following raw materials according to the ratio of weight and number, docetaxel: Brucea javanica oil: medium chain oil: soybean lecithin: polyethylene glycol-12-hydroxy hard Fatty acid ester: glycerin: sterile water for injection = 5: 150: 150: 30: 60: 35: 950.
[0035] 30g of refined soybean lecithin (Germany Lipoid company) and 60g of polyethylene glycol-12-hydroxystearate are dispersed in the composite oil phase composed of 150g of injection-grade javanica javanica oil and 150g of medium chain oil according to the above-mentioned weight ratio. Weigh 5g of docetaxel and add it to the above oil phase to dissolve, add 950g of sterilized water for injection at about 60°C and 35g of glycerin, and homogenize at 20000rpm for 2-4 minutes to form colostrum, and add the colostrum to the high-pressure homogenization Quality machine (Niro-Soavi, Italy), through N 2 Protection, the init...
Embodiment 3
[0038] The compound docetaxel lipid microsphere injection of the present invention is prepared from the following raw materials by weight and number: docetaxel: Brucea javanica oil: medium chain oil: lecithin: polyethylene glycol-12-hydroxystearin Ester: polyethylene glycol 1000 vitamin E succinate: glycerin: sterile water for injection = 2: 50: 50: 15: 7: 8: 20: 800.
[0039] Its preparation method is as follows:
[0040] Disperse 15g lecithin (German lipoid company) with 7g macrogol-12-hydroxystearate and 8g macrogol 1000 vitamin E succinate: dispersed in 50g injection grade Brucea javanica oil with 50 medium chain In the composite oil phase composed of oil, accurately weigh 2 g of docetaxel according to the above weight ratio and add it to the above oil phase to dissolve, add 800 g of sterilized water for injection at about 60 ° C and 20 g of glycerin, and homogenize at 20,000 rpm for 2-4 minutes , to form colostrum, the colostrum is added to the high-pressure homogenizer ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com