Transdermal medicine composition and transdermal medicine kit
A drug and preparation technology, which is applied in the field of transdermal drug combination preparations, can solve the problems of complex processing process and inability to use transdermal drug delivery, and achieve the effects of good release performance, excellent drug release effect, and reduced research and production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~8
[0050] Acrylamide and methylenebisacrylamide, acrylic acid and initiator azobisisobutyronitrile were added into a 100ml three-necked flask according to the amount described in Table 1, and 50ml of the first solvent (the first solvent used in Examples 1 to 7) was added. The first solvent is acetone, the first solvent used in Example 8 is isopropanol), a thermometer, a reflux device and a mechanical stirring device are installed, and the temperature is raised to 64° C. for reflux reaction under nitrogen protection for 30 hours. After removing the solvent by suction filtration, use Wash and purify the obtained polymer with ethanol until the content of acrylamide monomer in the cross-linked polyacrylamide obtained is lower than 0.000002% as measured by the detection method of the national standard GB / T12005.4-89, and then at 120°C Vacuum dried for 24 hours to obtain cross-linked polyacrylamide powder, and their glass transition temperature, cross-linking degree and gel skeleton por...
Embodiment 9
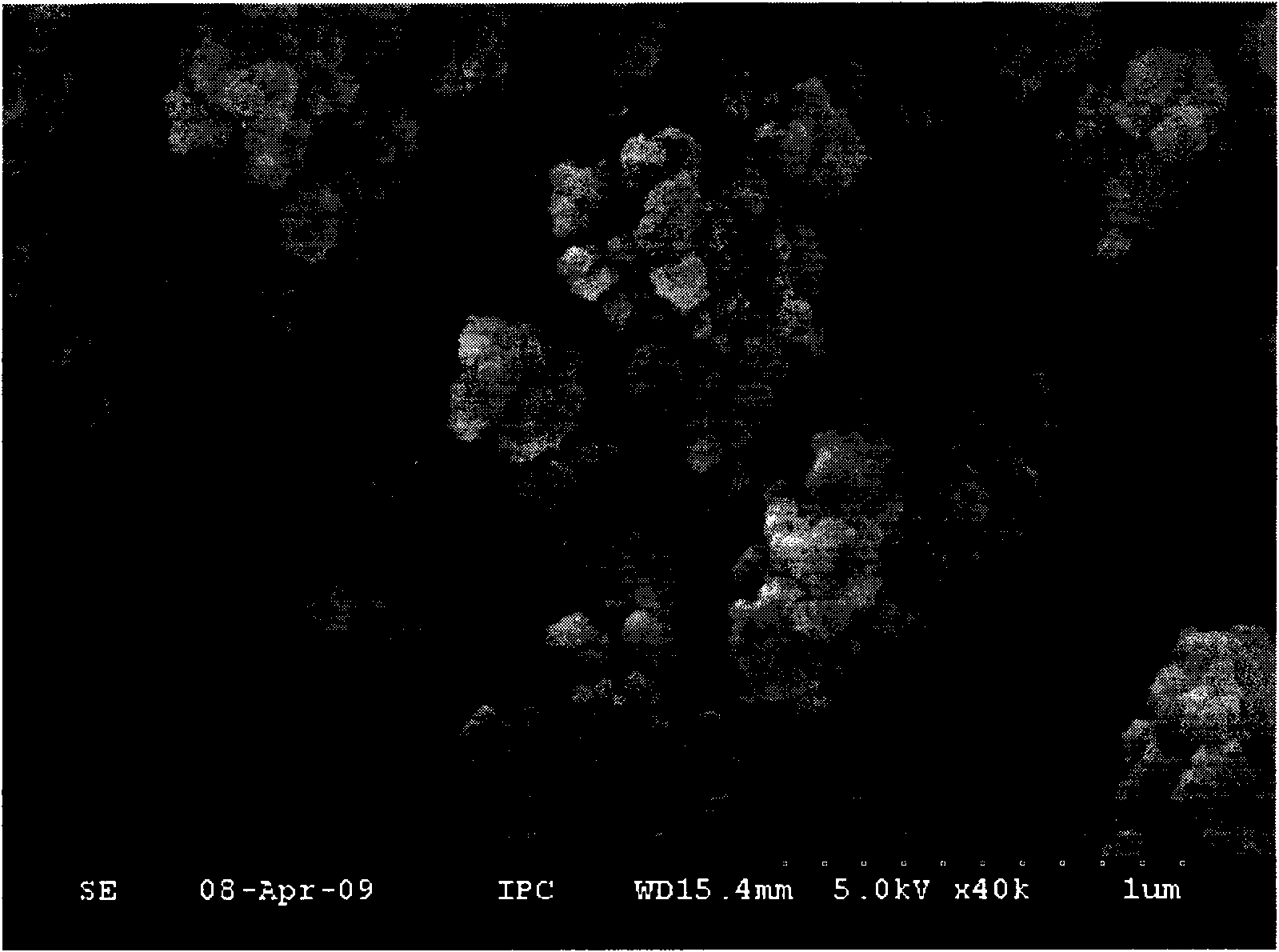
[0061] Take 0.038g of the cross-linked polyacrylamide powders of the above-mentioned Examples 1-8, add 1ml of aqueous solution, and after it swells into a gel, freeze-dry in a freeze-drying cold trap at -50°C for about 8 hours until the water in the sample The contents were lower than 10ppm, and the scanning electron microscope observation was carried out. The skeleton pore diameters of these gels after freeze-drying are listed in Table 1. figure 2 It shows the skeleton diagram of the gel formed by the cross-linked polyacrylamide powder in Example 2 after freeze-drying.
[0062] The results showed that the cross-linked polyacrylamide skeleton in these gels was observed to be porous when magnified 600 times, and the skeleton pore size was 5-30 μm. The cross-linked polyacrylamide powders of Examples 1-3 prepared according to the preferred conditions have a skeleton pore diameter of 12-30 μm after forming a gel.
Embodiment 10~22
[0067] The cross-linked polyacrylamide powders of the above-mentioned Examples 1 to 7 were formulated into different transdermal gel preparations according to the formula in Table 3 (the contents of each component are weight percentages based on the total amount of the gel preparation, and the remaining part is content of pure water), wherein the bovine serum albumin concentration is 2mg / g gel (bovine serum albumin molecular weight is 67kD, as can be seen from scanning electron microscope picture 2, the gel skeleton pore diameter is much larger than the size of various proteins, so It can be suitable for the release of proteins, polypeptides or gene medicines of various molecular weights), through the microneedle-assisted mode, use the microneedle array to act on the skin (484 holes) with an area of 1cm2 for 30s, and then inject 300mg of Example 10 ~22 The prepared hydrogel preparation was coated on the action site of the microneedle, and the classic Franz vertical jacketed d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com