Anti-D-dimer monoclonal antibody and application thereof
A monoclonal antibody, dimer technology, applied in the direction of anti-coagulation factor immunoglobulin, anti-animal/human immunoglobulin, biochemical equipment and methods, etc. Qualitative and other problems, to achieve the effect of good stability, high sensitivity and high diagnostic consistency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1: Preparation of anti-D-dimer monoclonal antibody
[0017] 1. Animal immunity
[0018] Take female BALB / c mice aged 6-8 weeks, and inject pure D-dimer with a concentration of 5mg / ml for the first immunization, and inject 1ml of the total amount through the abdominal cavity and under the armpit of the limbs; Methods Booster immunization once, a total of 3 immunizations, on the 4th day after the last immunization, blood was collected from the eyeballs of mice after three immunizations, the serum was separated by centrifugation, and mice with high titer were selected by ELISA method for fusion.
[0019] 2. Preparation of hybridoma cell lines
[0020] The splenocytes from the mice that had completed the immunization process were prepared to be fused with mouse myeloma cell SP2 / 0, and the feeder cells were prepared the day before the fusion; the mouse splenocytes were aseptically taken during fusion, and the splenocytes were mixed with SP2 / 0 cells at a ratio of 10:...
Embodiment 2
[0026] Example 2: Specific identification of monoclonal antibodies
[0027] Materials: Fibrinogen and its fragments X, Y, D, E and plasma.
[0028] Methods: ELISA method was used for detection, and D-dimer was used as positive control.
[0029] Results: Fibrinogen and its fragments X, Y, D, E and plasma were all negative.
[0030] Explanation: The anti-D-dimer monoclonal antibody of the present invention is highly specific to D-dimer.
Embodiment 3
[0031] Example 3: Cloning and sequencing of variable region sequences of monoclonal antibodies
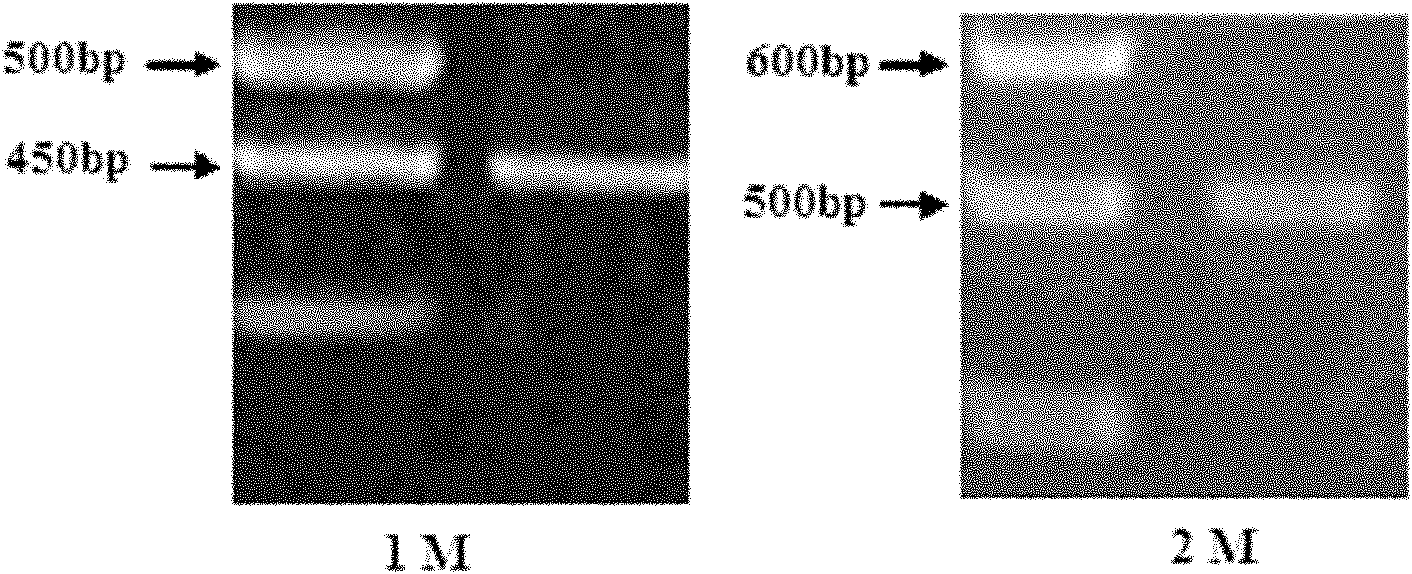
[0032] Using 5'RACE (Rapid Amplification of cDNA Ends, rapid amplification of cDNA ends) technology, the variable region sequence of the functional antibody was cloned from the hybridoma cell line 6B8D11H12 secreting anti-D-dimer monoclonal antibody. The steps can be briefly described as follows: an antisense gene-specific primer (GSP1) is used to synthesize the first strand of cDNA, after the first strand of cDNA is purified, terminal deoxynucleotidy transferase (Terminal deoxynucleotidy transferase, TdT) is used to generate A synthetic homopolynucleotide anchor sequence is added to the 3' end of the The cDNA is amplified using a second nested gene-specific primer (GSP2) and an anchor primer that anneals to the homopolynucleotide tail. The specific experimental process is as follows:
[0033] Material:
[0034] -Anti-D-dimer monoclonal antibody secreted by hybridoma cell line 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com